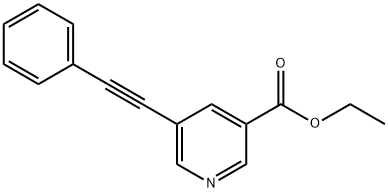
ETHYL 5-(2-PHENYLETH-1-YNYL)NICOTINATE synthesis
- Product Name:ETHYL 5-(2-PHENYLETH-1-YNYL)NICOTINATE
- CAS Number:175203-65-3
- Molecular formula:C16H13NO2
- Molecular Weight:251.28
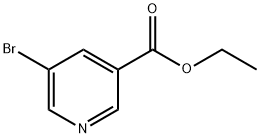
20986-40-7
272 suppliers
$6.00/10g
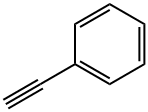
536-74-3
308 suppliers
$10.00/1g

175203-65-3
15 suppliers
inquiry
Yield:175203-65-3 100%
Reaction Conditions:
with copper(l) iodide;triethylamine;bis-triphenylphosphine-palladium(II) chloride in ethyl acetate at 50; for 20 h;Sonogashira Coupling;
Steps:
1.4
Step 4 - Representative procedure for the Sonagashira reaction of ethyl 5-bromonicotinate with acetylenes5-Phenylethynyl-nicotinic acid ethyl esterTo a solution of ethyl 5-bromonicotinate (1.15 g, 5 mmol) in ethyl acetate (20 mL) under an N2 atmosphere, was added triethylamine (1.1 mL, 7.5 mmol, 1.5 eq.), phenyl acetylene (0.766 g, 7.5 mmol, 1.5 eq.), dichloro-fe(triphenylphosphine)-palladium(II) (176 mg, 0.25 mmol, 0.05 eq.), and copper iodide (10 mg, 0.05 mmol, 0.01 eq). The reaction mixture was heated at 50 0C for 20 h before being cooled to room temperature, filtered through a pad of celite, and solvent evaporated to provide a dark brown oil. Silica gel column chromatography (9/1 - 4/1 hexanes/EtOAc elution) provided the title compound as a pale yellow oil (1.26 g, yield 100%). 1H NMR (300 MHz, CDCl3) δ 9.11 (d, J= 1.8 Hz, IH), 8.87 (d, J= 2.1 Hz, IH), 8.39 (dd, J=1.8, 2.1 Hz, IH), 7.56- 7.53 (m, 2H), 7.40-7.30 (m, 3H), 4.42 (q, J= 7.2 Hz, 2H), 1.43 (t, J= 7.2Hz, 3H); ESI-MS m/z 251.9 (M+H)+.
References:
WO2008/61236,2008,A2 Location in patent:Page/Page column 33
24242-19-1
259 suppliers
$6.00/1g

175203-65-3
15 suppliers
inquiry