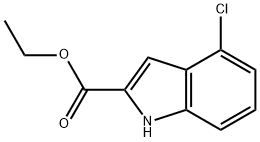
Ethyl-4-Chloroindole-2-Carboxylate synthesis
- Product Name:Ethyl-4-Chloroindole-2-Carboxylate
- CAS Number:53590-46-8
- Molecular formula:C11H10ClNO2
- Molecular Weight:223.66
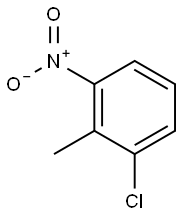
83-42-1
270 suppliers
$9.00/5g

7439-89-6
442 suppliers
$10.00/25g
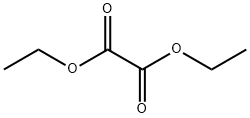
95-92-1
419 suppliers
$5.00/5 g

53590-46-8
86 suppliers
$44.00/100mg
Yield:-
Reaction Conditions:
with potassium tert-butylate in ethanol;dichloromethane;acetic acid
Steps:
3 Ethyl 4-chloroindole-2-carboxylate
Ethyl 4-chloroindole-2-carboxylate Potassium tert-butoxide (11.22 g, 0.1 mol) was added portionwise to stirred ethanol (25 mL) under Ar at ambient temperature. When the resultant viscous solution had cooled sufficiently, ether (300 mL) was added followed by diethyl oxalate (13.6 mL, 0.1 mol). After 10 min, 2-chloro-6-nitrotoluene (17.16 g, 0.1 mol) was added and the yellow solution became dark red. The reaction mixture was transferred to a conical flask and was stoppered and left standing at ambient temperature for 4 h, and was then transferred to the fridge for 65 h. The solid was collected by filtration, washed with ether until the filtrate ran colourless, and was sucked dry for 15 min. The isolated product (22.61 g, 73%) was used without further purification. To a solution of the above solid (11.2 g, 36.2 mmol) in acetic acid (250 mL) was added iron powder (7.08 g, 127 mmol) and the mixture was heated to 90 °C (external). As the external temperature reached ~ 90 °C an exotherm became apparent, with the internal temperature reaching 100 °C. The mixture became a light brown suspension, and after 15 min the exotherm had subsided. After a further 3 h at 90 °C the reaction was allowed to cool to 45 °C and was then poured into ice-water (500 mL). The mixture was extracted with ether (3 x 400 mL) and the combined extracts were washed with saturated aqueous sodium bicarbonate solution (repeated until effervescence ceased), water (400 mL), and 1N HCl (2 x 300 mL). The organic extracts were dried (magnesium sulfate) and the solvent removed in vacuo to afford the crude product as a yellow-orange oil (5.38 g). This material was dissolved in dichloromethane and passed down a short plug of silica. Removal of solvent afforded the title compound (4.38 g, 54%) as a pale-yellow solid: IR νmax (Nujol)/cm-1 3314, 2988, 2957, 2925, 2855, 1690, 1618, 1568, 1525, 1439, 1382, 1339, 1290, 1255, 1210, 1188, 1144, 1127, 1024, 977, 946, 822, 765, 674, 642, 598, 522 and 517; NMR δH (400 MHz; CDCl3) 1.43 (3H, t, J 7 Hz), 4.44 (2H, q, J 7 Hz), 7.16 (1H, dd, J 7.5, 1 Hz), 7.23 (1H, t, J 7.5 Hz), 7.32 (1H, dd, J 4.5, 1 Hz), 7.33 (1H, d, J 7 Hz).
References:
VERNALIS RESEARCH LIMITED EP1147110, 2003, B1
617-35-6
527 suppliers
$5.00/10g
108-42-9
462 suppliers
$10.00/5g
27034-51-1
146 suppliers
$6.00/250mg

53590-46-8
86 suppliers
$44.00/100mg
6361-22-4
134 suppliers
$17.00/250mg

1099-45-2
371 suppliers
$6.00/5g

53590-46-8
86 suppliers
$44.00/100mg

617-35-6
527 suppliers
$5.00/10g
2312-23-4
220 suppliers
$7.00/10g

53590-46-8
86 suppliers
$44.00/100mg
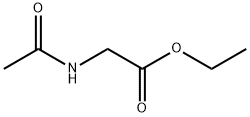
1906-82-7
192 suppliers
$13.00/5g
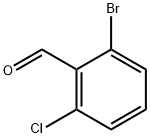
64622-16-8
147 suppliers
$7.00/250mg

53590-46-8
86 suppliers
$44.00/100mg