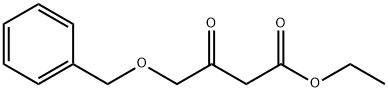
Ethyl 4-(benzyloxy)-3-oxobutanoate synthesis
- Product Name:Ethyl 4-(benzyloxy)-3-oxobutanoate
- CAS Number:67354-34-1
- Molecular formula:C13H16O4
- Molecular Weight:236.26
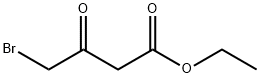
13176-46-0
164 suppliers
$8.00/250mg
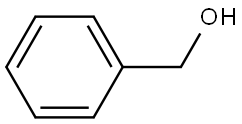
100-51-6
1396 suppliers
$5.00/100g

67354-34-1
86 suppliers
$30.00/1g
Yield: 88.5%
Reaction Conditions:
Stage #1:benzyl alcohol with sodium hydride in tetrahydrofuran at 0; for 0.5 h;Inert atmosphere;
Stage #2:4-bromoethyl acetoacetate in tetrahydrofuran at 20; for 12 h;
Steps:
1a
10149] To a stirred solution ofNaH (21.8 g, 912 mmol 3.0eq.) in THF (300 mL) was added BnOH (32.8 g, 304.0 mmol1.0 eq.) under a N2 atmosphere at 00 C. Afier addition, themixture was stirred for 30 mi Compound A (63.5 g, 304.0mmol 1.0 eq.) was added portionwise. The mixture wasallowed to warm to ambient temperature and stirred foranother 12 h. The reaction was monitored by TLC (petroleumether(PE):EtOAc=5:1). The mixture was poured into 2M HC1solution to .-pH 6. The solution was exacted with EtOAc (200mLx3). The combined organic phases were dried overNa2504, filtered and concentrated. The residue was purifiedby column chromatography on silica gel (PE:EtOAc=30: ito5:i)to give compoundB as a colorless oil (46 g, 88.5%). ‘HNMR (CDC13) ? 7.39-7.29 (m, 5H), 4.59 (s, 2H), 4.17-4.24(q, 2H), 4.14 (s, 2H), 3.53 (s, 2H), 1.31-1.22 (t, 3H).
References:
Hendricks, Robert Than;Beigelman, Leonid;Smith, David Bernard;Stoycheva, Antitsa Dimitrova US2015/72982, 2015, A1 Location in patent:Paragraph 0148; 0149
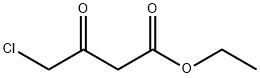
638-07-3
485 suppliers
$5.00/5 g

100-51-6
1396 suppliers
$5.00/100g

67354-34-1
86 suppliers
$30.00/1g

623-73-4
149 suppliers
$26.69/5g
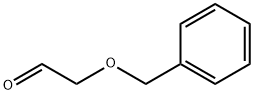
60656-87-3
176 suppliers
$17.00/1g

67354-34-1
86 suppliers
$30.00/1g
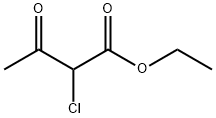
609-15-4
444 suppliers
$6.00/25g

100-51-6
1396 suppliers
$5.00/100g

67354-34-1
86 suppliers
$30.00/1g

638-07-3
485 suppliers
$5.00/5 g
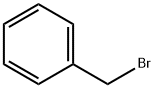
100-39-0
431 suppliers
$10.00/10 g

67354-34-1
86 suppliers
$30.00/1g