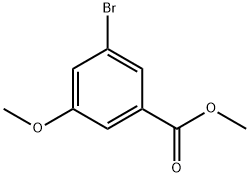
ethyl 3-bromo-5-methoxybenzoate synthesis
- Product Name:ethyl 3-bromo-5-methoxybenzoate
- CAS Number:56709-70-7
- Molecular formula:C9H9BrO3
- Molecular Weight:245.07
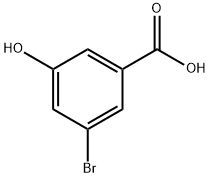
140472-69-1
170 suppliers
$19.00/250mg

74-88-4
346 suppliers
$15.00/10g

56709-70-7
56 suppliers
$250.00/1 G
Yield: 99%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 0 - 20; for 12 h;
Steps:
4.2. 3-Bromo-5-methoxybenzoic acid methyl ester (16)
To a mixture of 3-bromo-5-hydroxybenzoic acid (2.5 g, 11.52 mmol) and cesium carbonate (7.5 g, 23.04 mmol) in DMF (20 mL) was added methyl iodide (4.08 g, 28.8 mmol) at 0 °C. After 10 min the ice bath was removed and the reaction allowed to warm to room temperature. The reaction mixture was stirred at room temperature for 12 h. The reaction was quenched with water (50 mL) and extracted with methyl t-butyl ether (30 mL x 3), combined organic layer was dried over sodium sulfate. Concentration of the organic layer in vacuo followed by silica gel column chromatographic purification of the resulting residue using hexaneas an eluent gave the product 16 as a brown syrup (2.8 g, 99 %): 1H NMR (400 MHz, CDCl3) δ 7.75 (s, 1H), 7.48 (s, 1H), 7.23 (s, 1H), 3.91 (s, 3H), 3.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.69, 160.23, 132.69, 124.91, 122.70, 122.18, 113.47, 55.76, 52.48; IR (Neat) νmax 2952.04, 1727.14, 1678.80, 1286.68, 1049.20, 766.94 cm-1; MS: m/z 245.2 (M + H)+.
References:
Bhatthula, Bharath kumar goud;Kanchani, Janardhan reddy;Arava, Veera reddy;Subha [Tetrahedron,2019,vol. 75,# 7,p. 874 - 887]
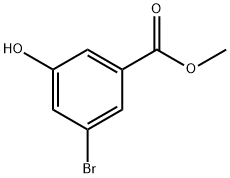
192810-12-1
88 suppliers
$72.90/250mg

74-88-4
346 suppliers
$15.00/10g

56709-70-7
56 suppliers
$250.00/1 G
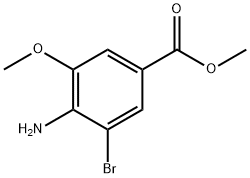
762292-57-9
13 suppliers
$145.00/500mg

56709-70-7
56 suppliers
$250.00/1 G

67-56-1
752 suppliers
$7.29/5ml-f
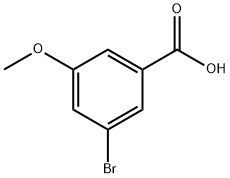
157893-14-6
115 suppliers
$10.00/250mg

56709-70-7
56 suppliers
$250.00/1 G

67-56-1
752 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
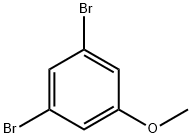
74137-36-3
214 suppliers
$8.00/1g

56709-70-7
56 suppliers
$250.00/1 G