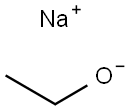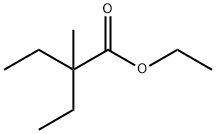
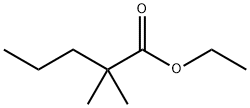
ethyl 2-ethyl-2-methylbutanoate synthesis
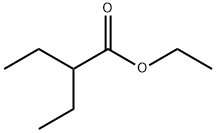
- Product Name:ethyl 2-ethyl-2-methylbutanoate
- CAS Number:19945-14-3
- Molecular formula:C9H18O2
- Molecular Weight:158.24
After dissolving ethyl 2-ethylbutyrate (50.0g, 346mmol) in 600mL tetrahydrofuran, N2 was bubbled into the resulting solution for 3 minutes, Then it was cooled to -78°C and then 190mL of 2M lithium diisopropylamide in tetrahydrofuran was added dropwise under N2 protection and -78°C, After the addition is complete, keep the reaction mixture at -78°C and continue to react for 30 minutes. Then iodomethane (58.9g, 415mmol) was slowly added to it, After the addition was completed, the reaction was slowly raised to room temperature overnight. Then slowly add saturated ammonium chloride solution to quench the reaction, and then separate the liquids. Collect the organic phase, extract the aqueous phase twice with dichloromethane, combine the organic phases, dry and spin dry to obtain the product. This is the desired ethyl 2-ethyl-2-methylbutanoate (52.2 g, yield 95%).

Yield:19945-14-3 95%
Reaction Conditions:
Stage #1: ethyl 2-ethylbutanoate in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: methyl iodide in tetrahydrofuran at 20;Inert atmosphere;
Steps:
1.1 Step 1: Synthesis of ethyl 2-ethyl-2-methylbutanoate
After dissolving ethyl 2-ethylbutyrate (50.0g, 346mmol) in 600mL tetrahydrofuran,N2 was bubbled into the resulting solution for 3 minutes,Then it was cooled to -78°C and then 190mL of 2M lithium diisopropylamide in tetrahydrofuran was added dropwise under N2 protection and -78°C,After the addition is complete, keep the reaction mixture at -78°C and continue to react for 30 minutes.Then iodomethane (58.9g, 415mmol) was slowly added to it,After the addition was completed, the reaction was slowly raised to room temperature overnight.Then slowly add saturated ammonium chloride solution to quench the reaction, and then separate the liquids.Collect the organic phase, extract the aqueous phase twice with dichloromethane, combine the organic phases, dry and spin dry to obtain the product.This is the desired ethyl 2-ethyl-2-methylbutanoate (52.2 g, yield 95%).
References:
CN111909212,2020,A Location in patent:Paragraph 0159-0162
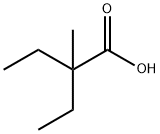
19889-37-3
54 suppliers
$285.00/250mg

19945-14-3
17 suppliers
inquiry
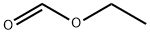
109-94-4
478 suppliers
$10.00/10g

111-27-3
510 suppliers
$5.00/25g
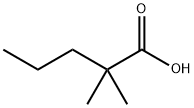
1185-39-3
98 suppliers
$17.00/250mg
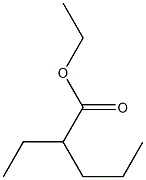
43164-26-7
3 suppliers
inquiry

19945-14-3
17 suppliers
inquiry
5837-93-4
0 suppliers
inquiry