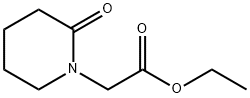
ethyl 2-(2-oxopiperidin-1-yl)acetate synthesis
- Product Name:ethyl 2-(2-oxopiperidin-1-yl)acetate
- CAS Number:22875-63-4
- Molecular formula:C9H15NO3
- Molecular Weight:185.22
Yield:22875-63-4 92%
Reaction Conditions:
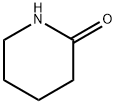
Stage #1: piperidin-2-onewith sodium hydride in tetrahydrofuran;mineral oil at 20; for 2 h;Inert atmosphere;
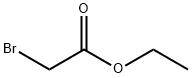
Stage #2: ethyl bromoacetate in tetrahydrofuran;mineral oil at 20; for 16 h;Inert atmosphere;
Steps:
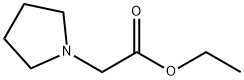
Ethyl 2-(2-oxopyrrolidin-1-yl)acetate (17)
General procedure: A solution of 2-pyrrolidinone (16, 5.00 g,58.8 mmol) in THF (50 mL) was added dropwise over 20 min to a stirred suspension ofNaH (60% dispersion in oil, 2.81 g, 70.3 mmol, 1.2 equiv) in THF (100 mL) under aninert atmosphere of Ar gas. The residual material in the dropping funnel was then rinsed into the reactionflask using additional THF (20 mL). Stirring was continued at room temperature for a further 2 h. Ethyl 2-bromoacetate (11.70 g, 70.1 mmol, 1.2 equiv) in THF (50 mL) was then added dropwise to the opaquewhite emulsion, and the reaction mixture was stirred under Ar at room temperature for 16 h. Water (100mL) was then cautiously added to the mixture until bubbling ceased and all solids dissolved. The mixturewas then extracted into EtOAc (100 mL). The organic phase was separated and the aqueous phase was reextractedwith EtOAc (2 × 50 mL). The combined organic phases were washed with brine (100 mL) anddried over anhydrous Na2SO4. The solvent was filtered and evaporated to give a crude yellow oil, whichwas purified using column chromatography (EtOAc) to afford lactam 17 (7.76 g, 77%) as a colorless oil;
References:
De Koning, Charles B.;Klintworth, Robin;Michael, Joseph P.;Morgans, Garreth L.;Scalzullo, Stefania M.;Van Otterlo, Willem A. L.0000-0002-3300-6463 [Beilstein Journal of Organic Chemistry,2021,vol. 17,p. 2543 - 2552] Location in patent:supporting information
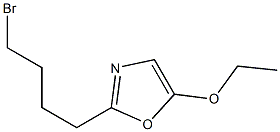
132353-41-4
0 suppliers
inquiry

22875-63-4
9 suppliers
inquiry
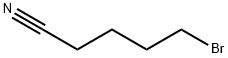
5414-21-1
158 suppliers
$10.00/5g

22875-63-4
9 suppliers
inquiry