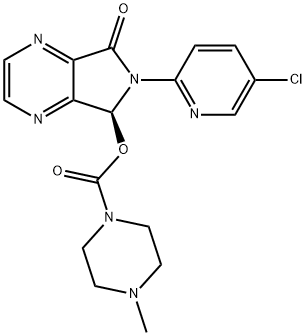
Eszopiclone synthesis
- Product Name:Eszopiclone
- CAS Number:138729-47-2
- Molecular formula:C17H17ClN6O3
- Molecular Weight:388.81

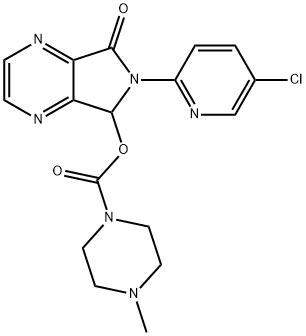
43200-80-2
4 suppliers
$71.00/1mg

138729-47-2
11 suppliers
$60.00/10mg
Yield:138729-47-2 91.7%
Reaction Conditions:
Stage #1: zopiclonwith D-Malic acid in methanol;acetone at 10 - 56; for 8.5 h;
Stage #2: with potassium carbonate in water; for 1 h;Solvent;Temperature;Reagent/catalyst;
Steps:
3 Preparation of eszopiclone
30 g (57.4 mmo 1) of eszopiclone D-malic acid salt intermediate prepared by the preparation method of Comparative Example 2, In the reaction flask, 40 g of zopiclone, 13.4 g of D-malic acid, 243 g of methanol, 413 g of acetone and the oil bath was heated to reflux (56 ° C) for 30 minutes and then slowly cooled to 10-15 ° C for 8 hours. suction and filtration carried out to obtain crude product. The crude salt, 168 g of methanol and 247 g of acetone were introduced into the reaction flask. The oil bath was heated to reflux (56 ° C) for 30 minutes and then slowly cooled to 10-15 ° C and allowed to stand for 8 hours. Acetone (2 * 20ml, 0-5 ° C) was used to wash filter cake , and dried under reduced pressure to give eszopiclone D-malic acid salt (21.9 g, 40.7%, 99.0% ee). Into the reaction bottle, add water 450g, open the stirring, until the eszopiclone D-malic acid salt dissolved, 15 g of povidone K30 was added and stirring was continued until the solid was dissolved. The stirring speed was adjusted to 80 m / min and poured into a 10 wt% aqueous solution of potassium carbonate95 g (68.8 mmol, 1.20 times of the drug), stirring was continued for 1 hour, filtered, the filter cake was washed three times with 90% of 1% aqueous potassium carbonate solution,The obtained wet crystals were dried at 60 ° C and 150 mmHg under reduced pressure for 8 hours to obtain 20.45 g of a white crystallite solid in a yield of 91.7%. Sampling analysis: the eszopiclonecontent of 99.6%, total impurities 0.04%, no ethyl acetate residue.
References:
CN103980278,2017,B Location in patent:Paragraph 0055-0060; 0063; 0068-0070; 0072; 0075; 0078
109-01-3
659 suppliers
$5.00/5G
![Carbonic acid, chloromethyl (5S)-6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester](/CAS/20210305/GIF/508169-20-8.gif)
508169-20-8
0 suppliers
inquiry

138729-47-2
11 suppliers
$60.00/10mg

43200-80-2
4 suppliers
$71.00/1mg
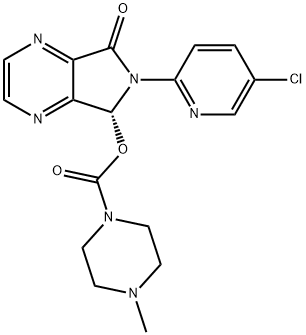
138680-08-7
1 suppliers
inquiry

138729-47-2
11 suppliers
$60.00/10mg
![Butanedioic acid, 2,3-bis[(4-methoxybenzoyl)oxy]-, (2R,3R)-, compd. with (5S)-6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methyl-1-piperazinecarboxylate (1:1)](/CAS/20211123/GIF/1225275-18-2.gif)
1225275-18-2
0 suppliers
inquiry

138729-47-2
11 suppliers
$60.00/10mg

138680-08-7
1 suppliers
inquiry

138729-47-2
11 suppliers
$60.00/10mg