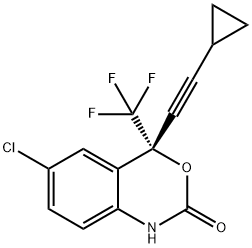
Enantiomer Efavirenz synthesis
- Product Name:Enantiomer Efavirenz
- CAS Number:154801-74-8
- Molecular formula:C14H9ClF3NO2
- Molecular Weight:315.67
32315-10-9
418 suppliers
$10.00/1g
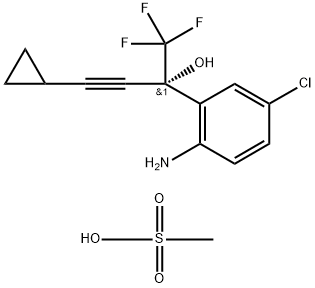
1236354-27-0
0 suppliers
inquiry

154801-74-8
43 suppliers
inquiry
Yield:154801-74-8 97.6%
Reaction Conditions:
Stage #1: (S)-2-(2-amino-5-chlorophenyl)-4-cyclopropyl-1,1,1-trifluorobut-3-yn-2-ol methanesulfonatewith sodium carbonate in n-heptane;water;ethyl acetate at 15; pH=6.1; for 0.333333 h;
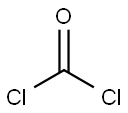
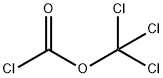
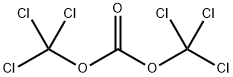
Stage #2: bis(trichloromethyl) carbonate at 12; for 1 h;Product distribution / selectivity;
Steps:
15
Aqueous Na2CO3 (14%-w/w, 80 g, 0.106 mol) was charged to SD573-MSA (50 g, 0.114 mol, corresponding to 33.4 g of SD573 free base) in ethyl acetate/heptanes (79.4 g, 1/1 v/v). After stirring for 15 min a pH of 6.1 was measured in the aqueous phase. The mixture was stirred for 5 min at 15 °C. Then the phase separation was performed and the aqueous phase was removed. The mixture was cooled to 12 °C and aqueous Na2CO3 (14% w/w, 135 g, 0.178 mol) was charged. To the biphasic mixture triphosgene in ethyl acetate (35.7%-w/w, 33.6 g, 40.5 mmol) was added in 60 min at 12 °C maximum. The mixture was stirred 30 min at 12°C maximum. Heptanes (48 g) were charged and a total conversion was obtained according to Method C. The reaction mixture was heated to 20 °C. Then a phase separation was performed and the aqueous phase was removed. The organic phase was washed with water (80 g) and then heated under reduced pressure to partially remove ethyl acetate, while heptanes were charged to the reaction mixture, to achieve a residual ethyl acetate content of 2.8%-w/w. A ratio of heptanes to organic matter of 9.5 L/kg in view of originally added SD573-MSA was obtained for crystallisation. The solution was seeded with DMP-266 (0.2 g) at 55 °C and stepwise cooled under stirring within 4 h 35 min to reach to -15 °C. The mixture was stirred overnight at -15 °C and then filtered. The filter cake was washed with pre-cooled heptanes (2×50 mL) at -10 °C maximum. The solid was dried in vacuo to yield 97.6% of DMP-266 (35.12 g, 111 mmol) at a purity of 95.1%-w/w according to Method D.
References:
EP2447247,2012,A1 Location in patent:Page/Page column 11
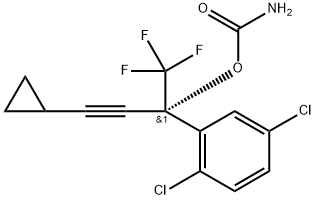
1381993-90-3
1 suppliers
inquiry

154801-74-8
43 suppliers
inquiry
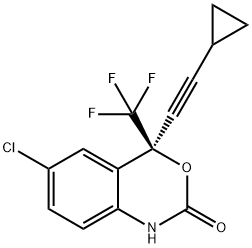
154598-52-4
495 suppliers
$25.00/1g

32315-10-9
418 suppliers
$10.00/1g
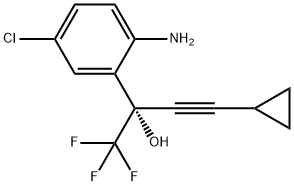
209414-27-7
125 suppliers
$55.00/10mg

154801-74-8
43 suppliers
inquiry