
Trabectedin synthesis
- Product Name:Trabectedin
- CAS Number:114899-77-3
- Molecular formula:C39H43N3O11S
- Molecular Weight:761.84
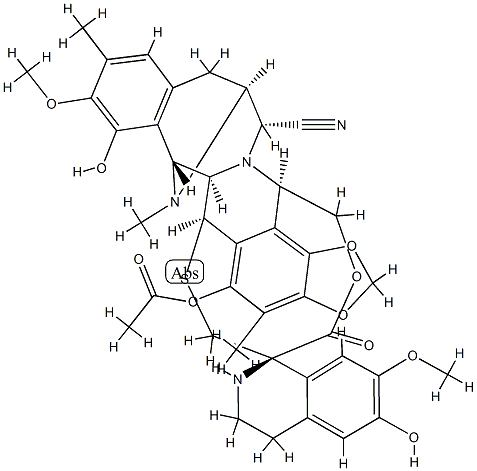
114899-80-8
39 suppliers
inquiry

114899-77-3
149 suppliers
$1000.00/5mg
Yield:114899-77-3 95%
Reaction Conditions:
with silver nitrate in water;acetonitrile at 20; for 24 h;Inert atmosphere;
Steps:
19 Example 19, Synthesis of Et-743
Compound 29 (7.7 mg, 0.01 mmol) was dissolved in 1 mL of acetonitrile in proportion, under argon.AgNO3 (32.3 mg, 0.19 mmol) was added to the mixed solvent of water, and the reaction was stirred at room temperature for 24 h.Add 1 mL of saturated sodium chloride and saturated sodium bicarbonate solution for 10 min.It was extracted with ethyl acetate three times, and the organic phases were combined, dried over anhydrous sodium sulfate and filtered.The solvent was removed by distillation under reduced pressure, flash column chromatography (DCM / MeOH = 20: 1), to give the compound 31 7.2mg (Et-743).The yield was 95%.
References:
CN109912629,2019,A Location in patent:Paragraph 0301-0305
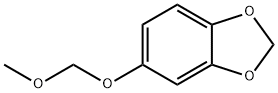
111726-43-3
18 suppliers
inquiry

114899-77-3
149 suppliers
$1000.00/5mg
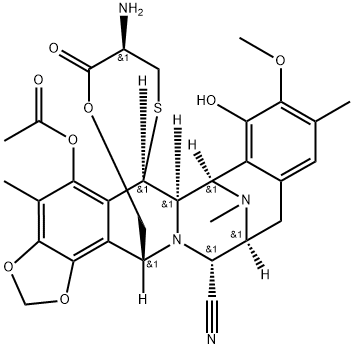
366020-68-0
10 suppliers
inquiry

114899-77-3
149 suppliers
$1000.00/5mg
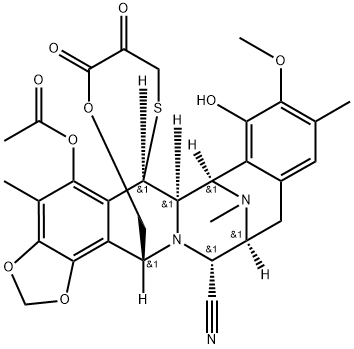
308359-33-3
14 suppliers
inquiry

114899-77-3
149 suppliers
$1000.00/5mg
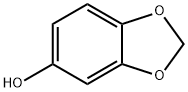
533-31-3
606 suppliers
$5.00/100mg

114899-77-3
149 suppliers
$1000.00/5mg