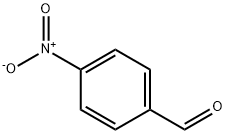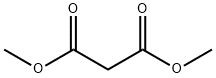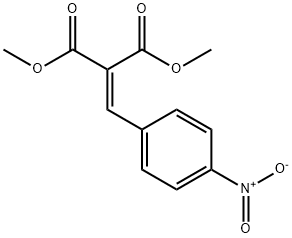
DIMETHYL (4-NITROBENZYLIDENE)MALONATE synthesis
- Product Name:DIMETHYL (4-NITROBENZYLIDENE)MALONATE
- CAS Number:38323-22-7
- Molecular formula:C12H11NO6
- Molecular Weight:265.22
Yield:38323-22-7 95%
Reaction Conditions:
with cross-linked polystyrene-titanium tetrachloride complex in neat (no solvent) at 60; for 4 h;Knoevenagel Condensation;
Steps:
2.1 2.5 Typical procedure for the solvent-free Knoevenagel reaction
General procedure: A mixture of aromatic aldehyde (1mmol), acetylacetone (6mmol) was charged into a 25ml of round-bottomed flask equipped with a magnetic stirrer, followed by adding PS/TiCl4 containing certain amount of TiCl4 (0.12g, 0.1mmol of TiCl4). The resulting mixture was heated at 60°C in a water bath with stirring for an appropriate period of time under solvent-free conditions (Table 1 ). The progress of the reaction was indicated by TLC (silica gel, petroleum ether or hexane/ethyl acetate). After completion of the reaction, the reaction mixture was allowed to cool to room temperature, and the catalyst was filtered and washed with ethyl acetate several times. The recovered catalyst was dried at 60°C for 2h. The washings were collected and combined with the filtrate. The organic solution was concentrated/evaporated under reduced pressure in a rotary evaporator, or treated by chromatography on a silica gel column (eluted by a mixture of petroleum ether and ethyl acetate), leading to the substituted olefin product with satisfactory purity.The structure of which was confirmed by m.p. data and with 1H NMR, and IR techniques. Characterization of the products was performed by comparison of their FT-IR, 1H NMR, and physical data with those of the authentic samples. Conversion was defined as a percentage of the starting aldehyde converted into products. The products were characterized by comparison of their physical data with those of the known samples or by comparison of their IR and NMR. The same procedure was applied for the condensation of β-ketoesters and dimethyl malonate with aromatic aldehydes (Table 3, entries 10-26). The characteristic data of selected compounds 3dk, 3dj are provided in supplementary data.
References:
Rahmatpour, Ali;Goodarzi, Niloofar [Catalysis Communications,2019,vol. 124,p. 24 - 31]