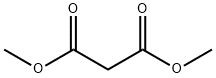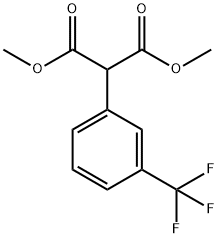
DIMETHYL-(3-(TRIFLUOROMETHYL)-PHENYL)-MALONATE synthesis
- Product Name:DIMETHYL-(3-(TRIFLUOROMETHYL)-PHENYL)-MALONATE
- CAS Number:138485-29-7
- Molecular formula:C12H11F3O4
- Molecular Weight:276.21
Yield:138485-29-7 75.8%
Reaction Conditions:
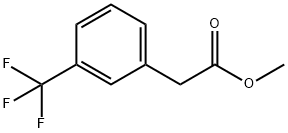
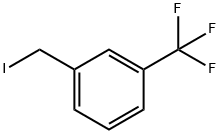
Stage #1: methyl 2-(3-(trifluoromethyl)phenyl)acetatewith sodium hydride in tetrahydrofuran;mineral oil; for 0.5 h;
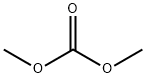
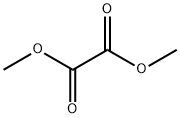
Stage #2: carbonic acid dimethyl ester in tetrahydrofuran;mineral oil at 60; for 18 h;
Steps:
Step 2: Synthesis of dimethyl 2-(3-(trifluoromethyl)phenyl)malonate
Methyl 2-(3-(trifluoromethyl)phenyl)acetate (2.9g, 17.5mmol) was dissolved in tetrahydrofuran (40mL), and then 60% sodium hydride (1.70g, 42mmol) was added in batches at room temperature, After the addition, the reaction was carried out at room temperature for 30 minutes; then dimethyl carbonate (8.1g, 90mmol) was dropped into the reaction, and the reaction was heated to 60°C after the dropping was completed and reflux for 18 hours. After the reaction was completed,15 mL of saturated ammonium chloride solution was added to quench the reaction,and then ethyl acetate (50 mL×3) was extracted. Combine the organic phases, dry with sodium sulfate for 1hour, filter,The filtrate was concentrated and separated by silica gelcolumn chromatography [petroleum ether/ethyl acetate (v/v)=10/1], the target product is obtained as a colorless liquid: 3.0g, with a yield of 75.8%.
References:
CN112851665,2021,A Location in patent:Paragraph 0150-0151
351-35-9
230 suppliers
$9.00/5g

138485-29-7
8 suppliers
inquiry