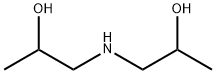
Diisopropanolamine synthesis
- Product Name:Diisopropanolamine
- CAS Number:110-97-4
- Molecular formula:C6H15NO2
- Molecular Weight:133.19
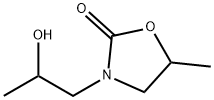
3375-84-6
22 suppliers
$45.00/50mg

110-97-4
299 suppliers
$13.00/25g
Yield:-
Reaction Conditions:
with potassium hydroxide in water at 80; for 2 h;Product distribution / selectivity;
Steps:
1; 2; 3; 4; 5; 6
These three examples illustrate actual laboratory experimentation on samples from three different locations with the intention of implementing the current invention's technology to perform the amine reclamation. Prior to testing, each amine solution was analyzed for % oxazolidone by gas chromatography, density, and % bound amine and % DIPA by acid/base titration.The following parameters are conditions under which these experiments were performed for each sample individually: An aliquot of the sample was selected and computations were made to determine the amounts of these listed species in that aliquot. This aliquot was placed into a 500-mL three necked boiling flask. A calculation was made to compute what amount of hydroxide (in the form of a 50% by weight potassium hydroxide solution) would be needed to present a ratio of 2.5 to 1 of hydroxide ions to reactable species. The amount of potassium hydroxide thus computed was added to the aliquot of amine in the boiling flask. A magnetic stirring bar was added to the flask, then the flask was placed in a hot water bath on top of a hot-plate stirring motor. A cold water condenser was placed into the center neck of the flask and a thermometer placed into one of the side necks of the flask. The third neck of the flask was capped with a stopcock. The flask's contents were vigorously stirred to completely mix the amine and caustic phases. The flask and the water bath were heated to approximately 80° C. (plus or minus 3° C.) and held at that temperature for approximately two hours while the solution was continuously stirred to ensure complete mixing contact of the amine and caustic phases. After the two hour mixing period was completed, the flask was removed from the heating and condensing apparatus and the solution was poured from the flask into a 500-mL separatory funnel. The phases were allowed to completely separate in the funnel and the bottom (caustic) phase was drawn off from the solution and segregated for subsequent analysis. The reaction product amine phase was analyzed for residual oxazolidone by gas chromatography, DIPA by acid/base titration, residual strong cations (as potassium) and excess hydroxide by acid/base titration.The reaction product caustic phase was analyzed for residual free hydroxide, carbonates and bicarbonates by acid/base titration but that information is not reported herein. This free hydroxide computation is important for the use of this phase as part of the subsequent batch's hydroxide source material.Tabulated results of the analysis of each amine phase are shown in Table 1. The results show a significant decrease in oxazolidone and a corresponding increase in DIPA concentration.; COMPARISON OF PRIOR ART AND THE PRESENT INVENTIONSamples of contaminated amine from various locations were obtained to compare the reduction of oxazolidone concentration in their process streams using the prior art and the new invention. These next two examples (example 4 in table 2 and example 5 in table 3) illustrate the contrast between the prior art and the predicted results using the present invention. The sixth example (table 4) contrasts expectations per the prior art and the actual observations during the commercial scale trial of the present invention.EXAMPLE 4System Size: 49,100 gallonsStarting Oxazolidone level: 20.4%;Targeted end value Oxazolidone level: 8.0%Oxazolidone incursion rate: 1 lbmol/day. The results show the predicted dramatic reduction in water usage, caustic usage, acid usage, effluent brine production, and reaction cycles, and processing time, when practicing the method of the present invention.; EXAMPLE 5System Size: 73,600 gallonsExisting Oxazolidone level: 23 wt %;Targeted end value Oxazolidone level: 8 wt %Oxazolidone incursion rate: 3 lbmol/day. The results show the predicted dramatic reduction in water usage, caustic usage, acid usage, effluent brine production, and reaction cycles, and processing time, when practicing the method of the present invention.; EXAMPLE 6System Size: 13,200 gallonsStarting Oxazolidone level: 37 wt %;Targeted end value Oxazolidone level: 4.4 wt %Oxazolidone incursion rate: 0.8 lbmol/day. The results show the actual dramatic reduction in water usage, caustic usage, acid usage, effluent brine production, and reaction cycles, and processing time, when practicing the method of the present invention.
References:
MPR Services, Inc. US7323600, 2008, B1 Location in patent:Page/Page column 5-8