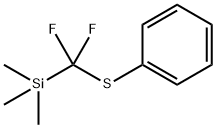
[[difluoro(triMethylsilyl)Methyl]thio]-Benzene synthesis
- Product Name:[[difluoro(triMethylsilyl)Methyl]thio]-Benzene
- CAS Number:536975-49-2
- Molecular formula:C10H14F2SSi
- Molecular Weight:232.37

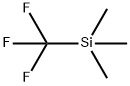
81290-20-2
408 suppliers
$13.00/1g
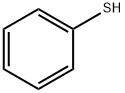
108-98-5
4 suppliers
$26.30/10g
![[[difluoro(triMethylsilyl)Methyl]thio]-Benzene](/CAS/GIF/536975-49-2.gif)
536975-49-2
18 suppliers
$100.00/100mg
Yield:536975-49-2 99%
Reaction Conditions:
Stage #1: thiophenolwith lithium tetrafluoroborate;lithium hydride in N,N-dimethyl-formamide at 25; for 0.0833333 h;Inert atmosphere;
Stage #2: (trifluoromethyl)trimethylsilane in N,N-dimethyl-formamide at 25;Inert atmosphere;
Steps:
[Difluoro(phenylsulfanyl)methyl]trimethylsilane (4)
Thiophenol (12.5g, 113.46mmol) is slowly added to a round-bottomed flask containing a mixture of LiBF4 (12.76g, 136.15mmol) and LiH (1.08g, 136.15mmol) in DMF (560mL). The resulting mixture was stirred for 5 min more under inert atmosphere. Addition of TMSCF3 (16.77mL, 113.46mmol) was followed by a vigorous stirring until the reaction went to completion (19F NMR check, needed approximately 15-30min). Then the reaction mixture is diluted with Et2O and filtered over a pad of silica. The filtered mixture is extracted (Et2O x3) and the organic layers collected and washed with water and brine. Removal of the organic solvent under vacuum gave the expected compound 4 (26.13g) in 99% yield. 1H NMR: δ=7.61 (m, 2H), 7.42-7.34 (massif, 3H), 0.23 (m, 9H). 13C NMR: δ=136.4 (t, J=1Hz), 134.3 (t, J=300Hz), 129.3, 128.9, 126.2 (t, J=4Hz), -4.0 (t, J=1Hz). 19F NMR: δ=-88.04 (s, 2F).
References:
Ismalaj, Ermal;Billard, Thierry [Journal of Fluorine Chemistry,2017,vol. 203,p. 215 - 217]
75-77-4
576 suppliers
$5.04/10
![Benzene, [(broModifluoroMethyl)thio]-](/CAS/20150408/GIF/78031-08-0.gif)
78031-08-0
4 suppliers
inquiry
![[[difluoro(triMethylsilyl)Methyl]thio]-Benzene](/CAS/GIF/536975-49-2.gif)
536975-49-2
18 suppliers
$100.00/100mg
![Benzene, [(broModifluoroMethyl)thio]-](/CAS/20150408/GIF/78031-08-0.gif)
78031-08-0
4 suppliers
inquiry
![[[difluoro(triMethylsilyl)Methyl]thio]-Benzene](/CAS/GIF/536975-49-2.gif)
536975-49-2
18 suppliers
$100.00/100mg
930-69-8
2 suppliers
$45.00/500mg
![[[difluoro(triMethylsilyl)Methyl]thio]-Benzene](/CAS/GIF/536975-49-2.gif)
536975-49-2
18 suppliers
$100.00/100mg

108-98-5
4 suppliers
$26.30/10g
![[[difluoro(triMethylsilyl)Methyl]thio]-Benzene](/CAS/GIF/536975-49-2.gif)
536975-49-2
18 suppliers
$100.00/100mg