
Dibutylamine synthesis
- Product Name:Dibutylamine
- CAS Number:111-92-2
- Molecular formula:C8H19N
- Molecular Weight:129.24
N-Nitrosamines and their precursors including n-dibutylamine are present in rubber products in which the accelerators and stabilizers used in the vulcanization process were derived from dialkylamines. Analysis of a single extraction of rubber nipples and baby pacifiers with artificial saliva (containing sodium nitrite) showed n-dibutylamine levels up to 3890 p.p.b. and N-nitrosodibutylamine concentrations as high as 427 p.p.b. (Thompson et al 1984).
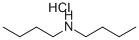
6287-40-7
82 suppliers
$18.00/10g

111-92-2
393 suppliers
$14.00/25mL
Yield:111-92-2 97%
Reaction Conditions:
with ammonia in 1,2-dichloro-benzene at 20;
Steps:
D
D) Recovery of the Secondary Amine; The recovery of the dibutylamine from the reaction mixture from C) was accomplished initially by filtration removal of the salt formed in C). The solid was slurried in dichlorobenzene, with addition of about 90% by weight of solvent. The resulting slurry was reacted with ammonia gas (20 liters/h) in a flask at 20° C., with thorough mixing by means of a stirrer, to form the amine and ammonium chloride. The yield of dibutylamine formed, based on salt, was 97%.
References:
US2013/6013,2013,A1 Location in patent:Page/Page column 6

109-74-0
5 suppliers
$20.00/25mL

111-92-2
393 suppliers
$14.00/25mL
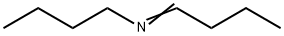
4853-56-9
5 suppliers
inquiry

111-92-2
393 suppliers
$14.00/25mL

109-65-9
547 suppliers
$10.00/10g

111-92-2
393 suppliers
$14.00/25mL

109-74-0
5 suppliers
$20.00/25mL
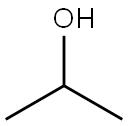
67-63-0
1581 suppliers
$17.00/25ML
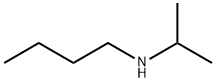
39099-23-5
11 suppliers
$60.00/100mg

111-92-2
393 suppliers
$14.00/25mL