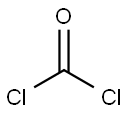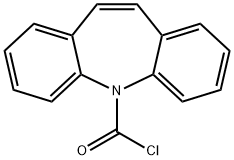
Dibenz[b,f]azepine-5-carbonyl chloride synthesis
- Product Name:Dibenz[b,f]azepine-5-carbonyl chloride
- CAS Number:33948-22-0
- Molecular formula:C15H10ClNO
- Molecular Weight:255.7
33948-19-5
193 suppliers
$8.00/100mg
![Dibenz[b,f]azepine-5-carbonyl chloride](/CAS/GIF/33948-22-0.gif)
33948-22-0
234 suppliers
$67.00/5g
Yield:33948-22-0 90.5%
Reaction Conditions:
with sodium bromate;N-benzyl-N,N,N-triethylammonium chloride;bromine;dibenzoyl peroxide in chlorobenzene at 92 - 97; for 8.5 h;Temperature;Reagent/catalyst;
Steps:
1 Example 1
A method for the synthesis of carbamazepine intermediate imidazolyl chloride, comprising the steps of:848 ml of chlorobenzene was added to the reactor,Iminodibenzyl chloride 106g (0.410mol),Benzoyl peroxide 4.24 g andBenzyltriethylammonium chloride 6.36 g,The oil bath was heated to 92 ° C,To the reactor was added sodium bromate 13.6 (0.09 mol)28.8 g (0.18 mol) of bromine was added dropwise,Dropping time of 4.5h,Then heated to 97 ° C and reflux for 4 h,After completion of the reflux, the reaction solution was cooled to 20 ° C,Poured into 300 ml of methanol solution,Static separation 4h, filtration, filter cake drying and vacuum distillation after evaporation of organic solvents, solid phase dissolved in 848ml toluene, adding activated carbon for recrystallization decolorization, filtration,Filtrate cooling crystallization, filtration,And dried in vacuo to give a yellow solid of about 95g imino stilbene chloride,The yield was 90.5%.
References:
Zhejiang Huazhou Pharmaceutical Co., Ltd.;Dai Chaoyun;Xu Weiling CN106467490, 2017, A Location in patent:Paragraph 0020; 0021; 0022; 0023; 0024; 0025; 0026-0035
256-96-2
292 suppliers
$5.00/100mg
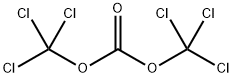
32315-10-9
418 suppliers
$10.00/1g
![Dibenz[b,f]azepine-5-carbonyl chloride](/CAS/GIF/33948-22-0.gif)
33948-22-0
234 suppliers
$67.00/5g
![5H-Dibenz[b,f]azepine-5-carbonyl chloride, 10-bromo-](/CAS/20200611/GIF/143667-53-2.gif)
143667-53-2
5 suppliers
inquiry
![Dibenz[b,f]azepine-5-carbonyl chloride](/CAS/GIF/33948-22-0.gif)
33948-22-0
234 suppliers
$67.00/5g
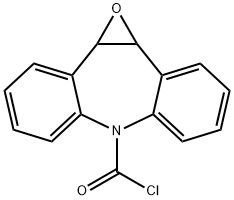
41359-09-5
10 suppliers
inquiry
![Dibenz[b,f]azepine-5-carbonyl chloride](/CAS/GIF/33948-22-0.gif)
33948-22-0
234 suppliers
$67.00/5g