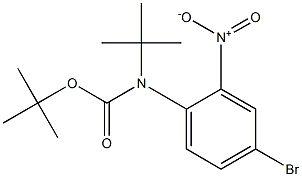
di-tert-butyl 4-bromo-2-nitrophenylcarbamate synthesis
- Product Name:di-tert-butyl 4-bromo-2-nitrophenylcarbamate
- CAS Number:1228392-59-3
- Molecular formula:C16H21BrN2O6
- Molecular Weight:417.25

24424-99-5
865 suppliers
$13.50/25G
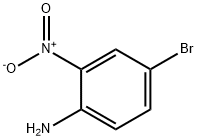
875-51-4
304 suppliers
$5.00/1g

1228392-59-3
24 suppliers
inquiry
Yield:1228392-59-3 90%
Reaction Conditions:
with dmap in tetrahydrofuran at 65;
Steps:
3 Synthesis of Bis(Boc)-4-bromo-2-nitroaniline
A 3-necked 5 L round bottom flask was charge with 4-bromo-3-nitroaniline (200g, 922 mmol), Boc anhydride (412g, 1889 mmol, 2.05 equiv), and THF (3L). To the stirred mixture was charged DMAP (1 1.26g, 92.2 mmol, 0.10 equiv). The mixture was heated to 65 °C and stirred at this temperature until the reaction was deemed complete by HPLC (< 2% 4-bromo-3- nitroaniline remaining, ca. 3.5 hours) and then cooled to room temperature. The mixture was transferred to a 12 L workup vessel, ethyl acetate was added (3 L) and the mixture was washed sequentially with with I N HC1 (1 L), saturated aqueous NaHC03 solution (1 L), and 10% aqueous NaCl solution (1 L). The organic layer was concentrated to a minimum stirrable volume and ethyl acetate (1 L) was added. The solution was chased twice with heptane ( 1.5 L), concentrated the mixture to a total volume of 1.5 L following the second chase. The resulting slurry was filtered, washed with heptane (3 x 200 mL), and dried under vacuum to provide the title compound (345.5g, 90% yield) as an off-white solid.
References:
WO2016/168331,2016,A1 Location in patent:Page/Page column 26-27