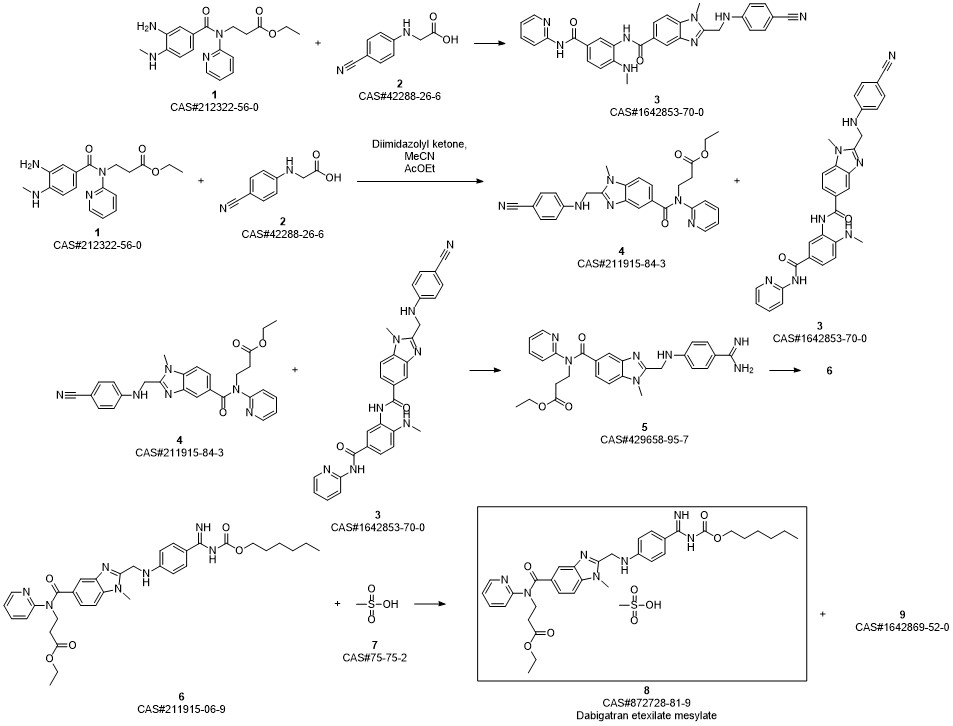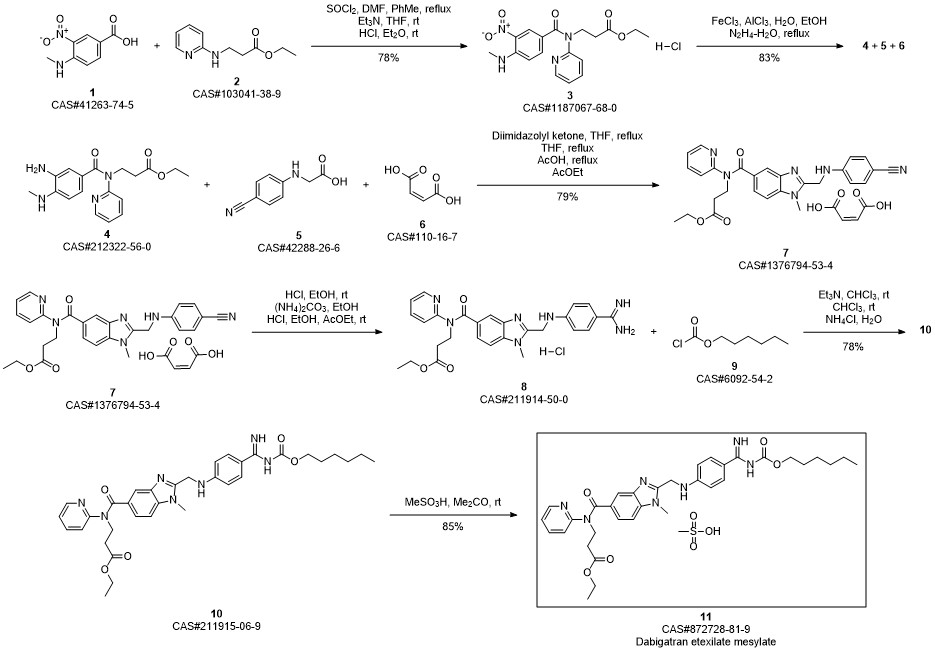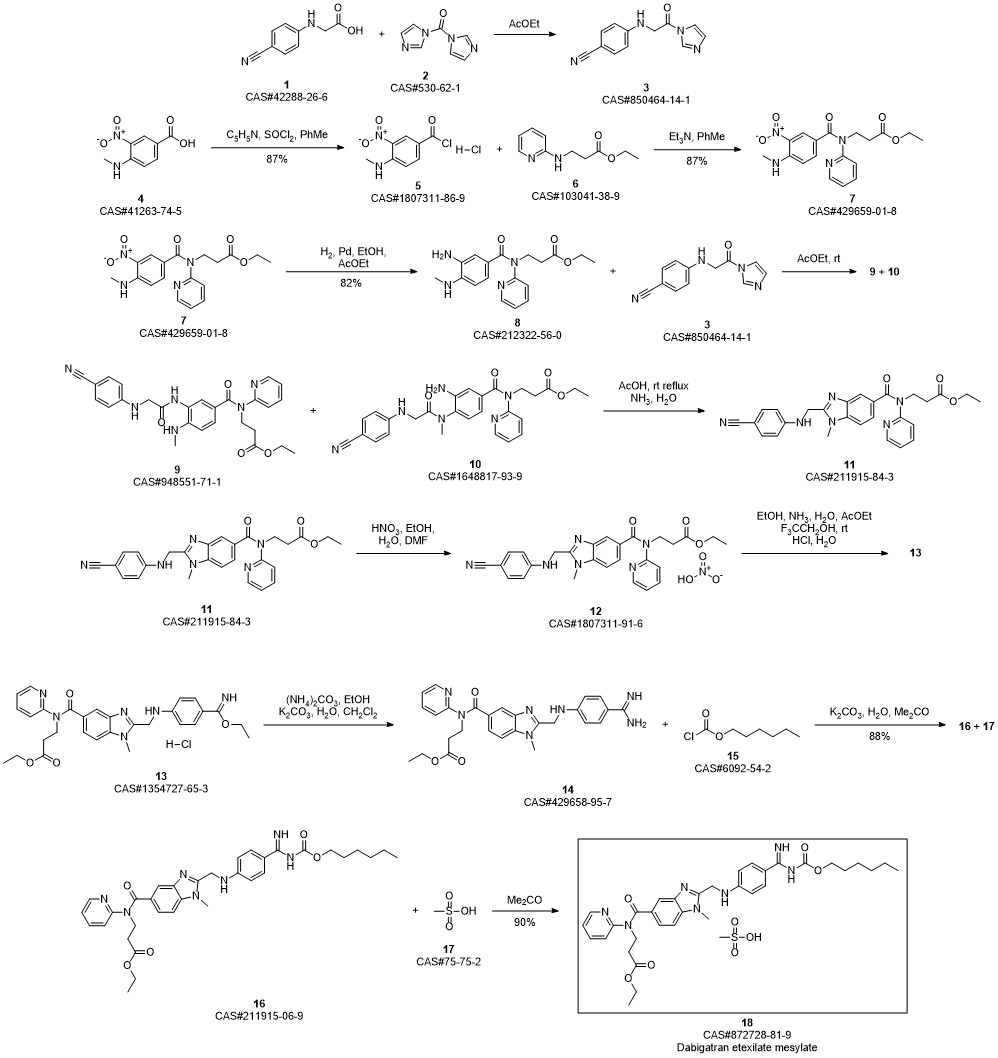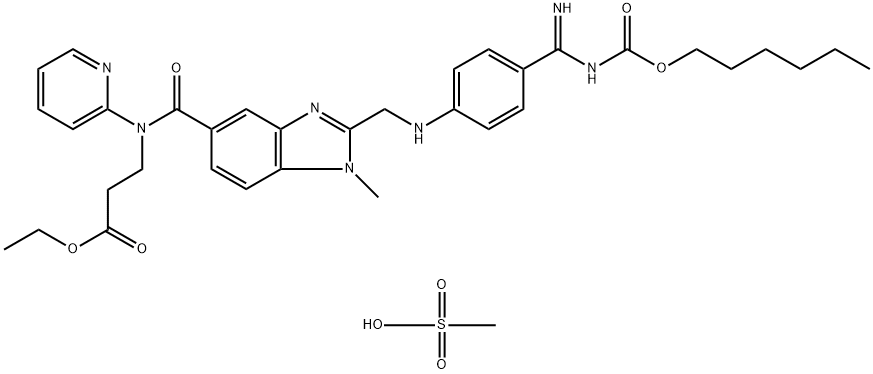
Dabigatran Etexilate Mesylate synthesis
- Product Name:Dabigatran Etexilate Mesylate
- CAS Number:872728-81-9
- Molecular formula:C35H45N7O8S
- Molecular Weight:723.85
Devarasetty, Sitaramaiah; Janni, Ravi; Nunna, Rambabu; Suraparaju, Raghu Ram. An improved process for the preparation of Dabigatran Etexilate mesylate and synthesis of its impurities. Pharma Chemica. Volume 10. Issue 4. Pages 127-148. Journal; Online Computer File. (2018).
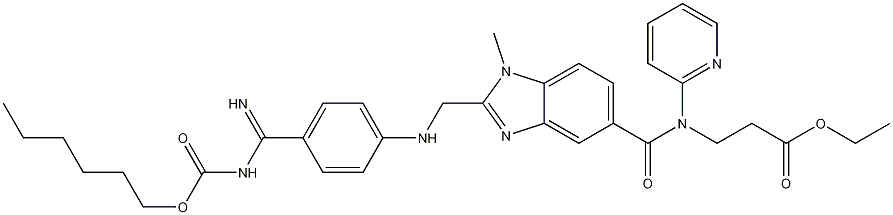
211915-06-9
416 suppliers
$5.00/50mg
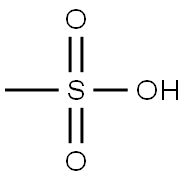
75-75-2
629 suppliers
$10.00/5g

872728-81-9
448 suppliers
inquiry
Yield:872728-81-9 98%
Reaction Conditions:
in acetone at 26 - 36;Industry scale;
Steps:
1
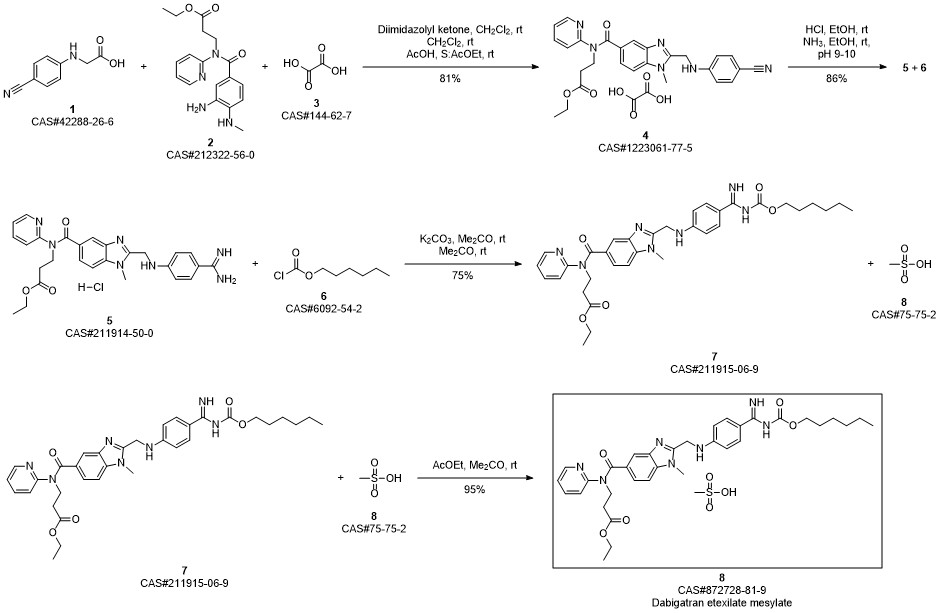
Ethyl 3 - [(2- { [4-(hexyloxycarbonylarninoimmomemyl)phenylammo]methyl } -1 - methyl- lH-benzimidazole-5-carbonyl)pyridm-2-ylamino]propionate base (52.6 kg) (which has preferably been purified beforehand by recrystallization from ethyl acetate) is placed in an agitator apparatus which has been rendered inert and then 293 kg of acetone is added. The contents of the apparatus are heated to 40° C to 46° C with stirring. After a clear solution has formed, the contents of the apparatus is filtered into a second agitator apparatus through a lens filter and then cooled to 30° C to 36° C. 33 kg of acetone precooled to 0° C to 5° C, 7.9 kg of 99.5% methanesulfonic acid, and for rinsing another 9 kg of acetone are placed in the suspended container of the second apparatus. The contents of the suspended container are added in metered amounts to the solution of ethyl 3-[(2-{[4-(hexyloxycarbonylamino- iminomethyl)phenylamino]methyl} - 1 -methyl- 1 H-benzimidazole-5-carbonyl)pyridin-2- ylamino]propionate base at 26° C to 36° C within 15 to 40 minutes. Then the mixture is stirred for 40 to 60 minutes at 26° C to 33° C. It is then cooled to 17° C to 23° C and stirred for a further 40 to 80 minutes. The crystal suspension is filtered through a filter dryer and washed with a total of 270 L of acetone. The product is dried in vacuum at a maximum of 50° C for at least 4 hours. Yield: 54.5-59.4 kg;90%-98% of theory based on ethyl 3-[(2-{[4-(hexyloxycarbonyl- ammoiminomethyl)phenylamino]methyl} - 1 -methyl- 1 H-benzimidazole-5-carbonyl)- pyridm-2-ylamino]propionate base.
References:
WO2012/44595,2012,A1 Location in patent:Page/Page column 15
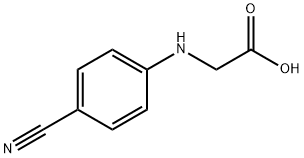
42288-26-6
553 suppliers
$14.00/1g

872728-81-9
448 suppliers
inquiry
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
536 suppliers
$14.00/1/ G

872728-81-9
448 suppliers
inquiry
![N-[[2-[[[4-(Aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]arbonyl]-N-2-pyridinyl-beta-alanine ethyl ester 4-methylbenzenesulfonate](/CAS/GIF/872728-85-3.gif)
872728-85-3
71 suppliers
inquiry

872728-81-9
448 suppliers
inquiry
![3-[[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]pyridin-2-ylamino]propionic acid ethyl ester](/CAS2/GIF/211915-84-3.gif)
211915-84-3
323 suppliers
inquiry

872728-81-9
448 suppliers
inquiry