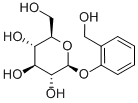
D-(-)-Salicin synthesis
- Product Name:D-(-)-Salicin
- CAS Number:138-52-3
- Molecular formula:C13H18O7
- Molecular Weight:286.28
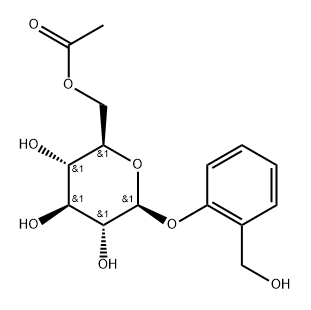
![2-[(Acetyloxy)methyl]phenyl β-D-glucopyranoside 2,3,4,6-tetraacetate](/CAS/GIF/16643-37-1.gif)
16643-37-1
0 suppliers
inquiry

138-52-3
476 suppliers
$6.00/1g
Yield:138-52-3 80 mg
Reaction Conditions:
with sodium methylate in methanol at 20; for 1 h;Inert atmosphere;
Steps:
Glucosides 24-28 General procedure
General procedure: To a solution 2 and the acceptor ArOH (3.0 equiv) in dry DCM (0.1 M) was added afreshly prepared solution of BF3?OEt2 (1.0 equiv., 1M in dry DCM). The solution wasstirred 1 h and followed by TLC (30:70; EtOAc : hexanes). A solution of NaHCO3(aq) wasadded and the mixture was extracted with DCM, washed with brine, dried over Na2SO4,filtered and concentrated in vacuo. The α/β ratio was measured by 1H NMR on the crude.The residue was treated with a freshly prepared solution MeONa (0.25 equiv., 0.5 M inmethanol) in dry methanol (0.3 M). The solution was stirred at r.t. for 1 h andneutralized with Amberlite IR-120 H+, filtered and concentrated in vacuo to give a whitesolid. Quick flash column chromatography (10:90; MeOH:DCM) removed excess of ArOHand led to the β-D anomer, which was recrystallized in hot ethanol.
References:
St-Pierre, Gabrielle;Dafik, Laila;Klegraf, Ellen;Hanessian, Stephen [Synthesis,2016,vol. 48,# 20,p. 3575 - 3588] Location in patent:supporting information
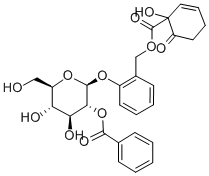
19764-02-4
2 suppliers
inquiry

138-52-3
476 suppliers
$6.00/1g
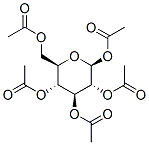
29836-40-6
14 suppliers
inquiry

138-52-3
476 suppliers
$6.00/1g
604-69-3
380 suppliers
$10.00/25g

138-52-3
476 suppliers
$6.00/1g
60523-66-2
0 suppliers
inquiry

138-52-3
476 suppliers
$6.00/1g