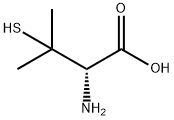
D-Penicillamine synthesis
- Product Name:D-Penicillamine
- CAS Number:52-67-5
- Molecular formula:C5H11NO2S
- Molecular Weight:149.21
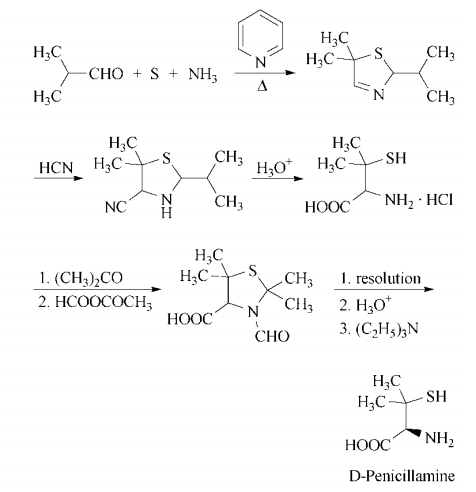
106-50-3
613 suppliers
$19.00/25g

52-67-5
335 suppliers
$5.00/50mg
Yield:52-67-5 47%
Steps:
20 Example 20
By substituting p-phenylenediamine for 2-naphthylamine in the above procedure, D-penicillamine was obtained in a yield of 47.0%.
References:
Taisho Pharmaceutical Co., Ltd. US4150240, 1979, A
137-07-5
302 suppliers
$10.00/5g

52-67-5
335 suppliers
$5.00/50mg
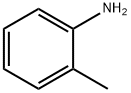
95-53-4
435 suppliers
$10.00/1g

52-67-5
335 suppliers
$5.00/50mg
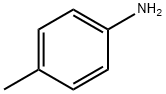
106-49-0
397 suppliers
$5.00/5 g

52-67-5
335 suppliers
$5.00/50mg