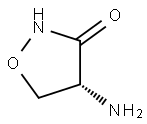
D-Cycloserine synthesis
- Product Name:D-Cycloserine
- CAS Number:68-41-7
- Molecular formula:C3H6N2O2
- Molecular Weight:102.09
144533-21-1
0 suppliers
inquiry

68-41-7
542 suppliers
$5.00/100mg
Yield:68-41-7 315 mg
Reaction Conditions:
with water;sodium hydroxide in methanol;dichloromethane at 20; for 4 h;
Steps:
(c) Synthesis of compound 6 from compound 3:
Sodium methoxide (12.27 mmol) in MeOH was added to a solution of hydroxylamine hydrochloride (465 mg, 6.69 mmol) in MeOH (10 mL). The mixture was stirred at room temperature for 30 min and cooled to 0 °C. Then the sodium chloride was filtered. A solution of compound 3 (1.2 g, 5.58 mmol) in CH3CN (20 mL) was added to the filtrate at 0 °C. The mixture was stirred at 0 °C for 4 h and concentrated under reduced pressure. Et3N (1.16 mL, 8.37 mmol) and CH2Cl2 (30 mL) was added to the mixture and stirred at 0 °C for 10 min. Methanesulfonyl chloride (0.73 g, 6.14 mmol) was added next, and the mixture was stirred at 0 °C for 1 h. DBU (1.27 g, 8.37 mmol) was added to the mixture at 0 °C and stirred at room temperature for 2 d. After NaOH (1.12 g, 27 mmol) in water (20 mL) and MeOH (20 mL) were added, the mixture was stirred at room temperature for 4 h. 50 mL of ethanol/isopropyl alcohol was added. The precipitate salts were filtered, and the filtrated was cooled to 0 °C in the ice bath. Glacial acetic acid was added dropwise to the well-stirred mixture to reach pH 6.0 and gave a colorless solid. The crystalline precipitate was filtered and washed twice with 1:1 ethanol/isopropyl alcohol and diethyl ether to give d-4-amino-3-isoxazolidinone (315 mg, 55%). mp 146-148 °C; +110 (c 1.0, H2O); 1H NMR (DMSO-d6, 300 MHz) δ 4.38 (t, 1H), 3.51 (m, 2H); 13C NMR (DMSO-d6, 75 MHz) δ 174.5, 75.1, 53.6; HRMS (ESI) m/z (M+H)+ calcd for C3H6N2O2 = 102.0919, found 102.0743.
References:
Kim, Hee-Kwon;Park, Kyoung-Joo Jenny [Tetrahedron Letters,2012,vol. 53,# 13,p. 1668 - 1670] Location in patent:experimental part
17136-54-8
108 suppliers
$32.00/250mg

68-41-7
542 suppliers
$5.00/100mg
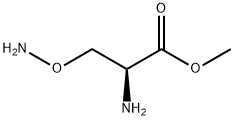
29491-80-3
1 suppliers
inquiry

68-41-7
542 suppliers
$5.00/100mg
![2-Isoxazolidinecarboxylic acid, 3-oxo-4-[[(phenylmethoxy)carbonyl]amino]-, phenylmethyl ester, (R)- (9CI)](/CAS/20210305/GIF/32296-75-6.gif)
32296-75-6
0 suppliers
inquiry

68-41-7
542 suppliers
$5.00/100mg
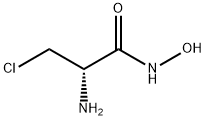
23628-35-5
2 suppliers
inquiry

68-41-7
542 suppliers
$5.00/100mg