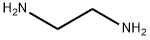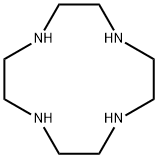
Cyclen synthesis
- Product Name:Cyclen
- CAS Number:294-90-6
- Molecular formula:C8H20N4
- Molecular Weight:172.27
TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4
The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine.
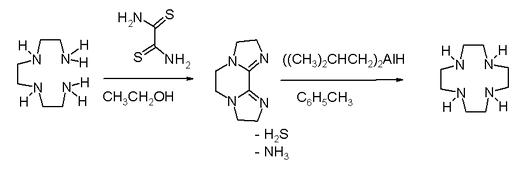
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL.
In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry.
wiki/Cyclen
10045-25-7
200 suppliers
$5.00/250mg

294-90-6
534 suppliers
$5.00/1g
Yield:294-90-6 95%
Reaction Conditions:
with hydrogenchloride
Steps:
1-10 1,4,7-tetraazacyclododecane
Dowex TM 1 × 8 200-400 mesh strong basic type I anion exchange resin (Cl type) was placed in a 300 mL beaker and dissolved in 1 M HCl.After filtering this with Nutche, the obtained filtrate was dissolved in distilled water and poured into a chromatographic tube filled with cotton and sea sand.After adding 1 M HCl until the pH of the droplet became 1, the pH was adjusted to 5 with distilled water. Then add 1 M NaOH until the pH reaches 12 and thenThe pH was adjusted to 7 using distilled water. A solution prepared by dissolving 1,4,7,10-tetraazacyclododecane tetrahydrochloride (1.50 g, 4.72 mmol) in the smallest amount of distilled water was put therein, and droplets having a basic pH were collected.This solution is concentrated on a rotary evaporator andVacuum drying gave a yellow solid. Dissolve this in CHCl3, add Na2SO4 to dehydrate it, filter it with Nutche, wash it with a small amount of CHCl3, collect the filtrate, and concentrate it with a rotary evaporator.Vacuum dried and left in the refrigerator to give a yellow solid(0.772 g, Yield 95%).
References:
JP2021/42186,2021,A Location in patent:Paragraph 0058-0059
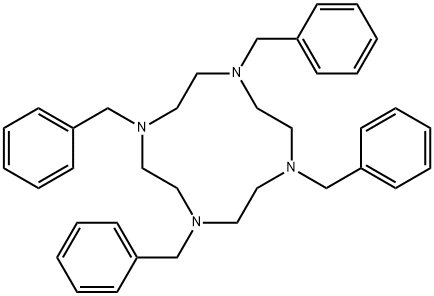
18084-64-5
100 suppliers
$39.00/1g

294-90-6
534 suppliers
$5.00/1g
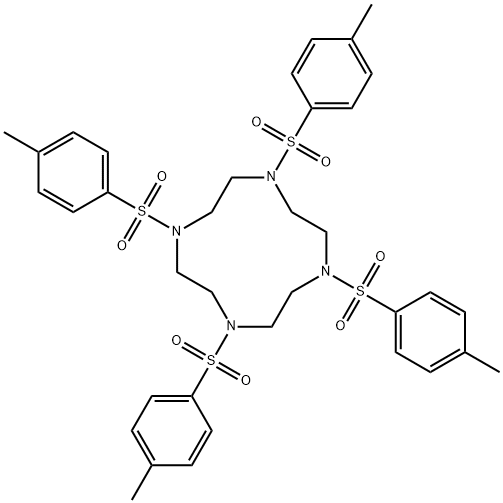
52667-88-6
61 suppliers
$480.00/1g

294-90-6
534 suppliers
$5.00/1g