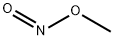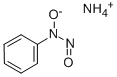
Cupferron synthesis
- Product Name:Cupferron
- CAS Number:135-20-6
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15
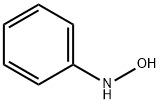
100-65-2
112 suppliers
$76.00/1g

135-20-6
343 suppliers
$20.00/25g
Yield:135-20-6 3.87 g
Reaction Conditions:
with n-Butyl nitrite;ammonia in diethyl ether at -10 - 0; for 0.25 h;
Steps:
1. General procedure for the preparation of cupferron and its derivatives
General procedure: The corresponding nitrobenzene (1 molar equiv., see below for specific details) was dissolved in 100 mL of 40% aqueous methanol (v/v) in a 250 mL reaction flask. To the solution was added ammonium chloride, 1 molar equiv.), and the solution was stirred at 40 °C followed by the addition of zinc dust (2 molar equiv.) portion wise with vigorous stirring for about 2.5 h. The progress of the reaction was monitored by TLC (hexanes/ethyl acetate, 90:10 (v/v)). The mixture was allowed to cool to ambient temperature and 50 mL of cold water was added. The solids were filtered off and washed with ether (50 mL), while the aqueous layer was extracted with ether (3 × 100 mL). The combined organic layer was dried over sodium sulphate. Thereafter, the ether solution was transferred to a reaction flask and was cooled in an ice-salt bath to -10 °C. Anhydrous ammonia gas from a compressed gas cylinder was bubbled through the solution for 15 minutes, after which the addition of ammonia was continued while butyl nitrite (1 molar equiv.) was added slowly over 10 minutes with stirring. The reaction mixture was stirred for another 15 minutes after the addition of butyl nitrite to ensure completion of the nitrosation reaction. The solid material was collected using a Buchner funnel, washed with ice cold ether and vacuum dried, to obtain the corresponding cupferron.
References:
Isa, Mustafa A.;Muller, Alfred;Sonopo, Molahlehi;Waziri, Ibrahim;Williams, D. Bradley G. [Bioorganic and Medicinal Chemistry Letters,2021,vol. 52,art. no. 128381] Location in patent:supporting information
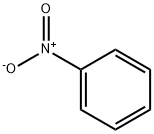
98-95-3
12 suppliers
$14.00/25g

135-20-6
343 suppliers
$20.00/25g

100-65-2
112 suppliers
$76.00/1g
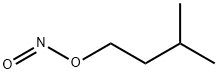
110-46-3
281 suppliers
$10.00/10g

135-20-6
343 suppliers
$20.00/25g