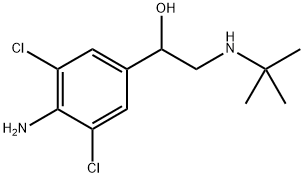
CLENBUTEROL synthesis
- Product Name:CLENBUTEROL
- CAS Number:37148-27-9
- Molecular formula:C12H18Cl2N2O
- Molecular Weight:277.19
37148-47-3
220 suppliers
$75.00/5mg
75-64-9
429 suppliers
$10.00/10g

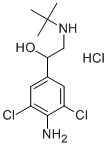
37148-27-9
101 suppliers
$60.00/2mg
Yield:37148-27-9 35%
Reaction Conditions:
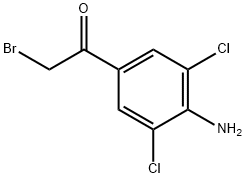
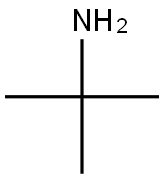
Stage #1: 1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one;tert-butylamine in tetrahydrofuran;ethanol at 0 - 20; for 4 h;Inert atmosphere;
Stage #2: with potassium borohydride in tetrahydrofuran;ethanol; for 2 h;Cooling with ice;
Stage #3: with methanol in tetrahydrofuran;ethanol at 20; for 16 h;
Steps:
1 Synthesis of Clenbuterol from 4-Amino-3,5-dichlorobromoacetophenone
Weigh 5.0 g (17.8 mmol) of 4-amino-3,5-dichlorobromoacetophenone in a 250 ml round bottom flask, add 50 ml of tetrahydrofuran (THF) and 50 ml of ethanol (CH3CH2OH), place in an ice water bath, and stir. Dissolve, deoxidize the device, protect with N2 gas. After 20 minutes, draw 3.8 ml (35.6 mmol) of t-butylamine with a syringe and slowly add to the reaction device.The reaction was continued at 0 ° C for 3 h and at room temperature for 1 h.Then place the reaction unit in an ice water bath.Weigh 1.5g (28mmol) of KBH4 and slowly add it to the reaction unit. After 2h,The ice bath was removed and 50 ml of methanol (CH3OH) was added to the reaction apparatus.Stir at room temperature for 16 h, and remove most of the solvent by rotary evaporation.The reaction was quenched by the addition of 30 mL of water and extracted three times with dichloromethane.The organic phases were combined and dried over anhydrous sodium sulfate.After filtration, the solvent was removed, and 1.7 g of Clenbuterol was obtained by column chromatography.The yield was 35%.
References:
CN109912434,2019,A Location in patent:Paragraph 0038; 0045-0050
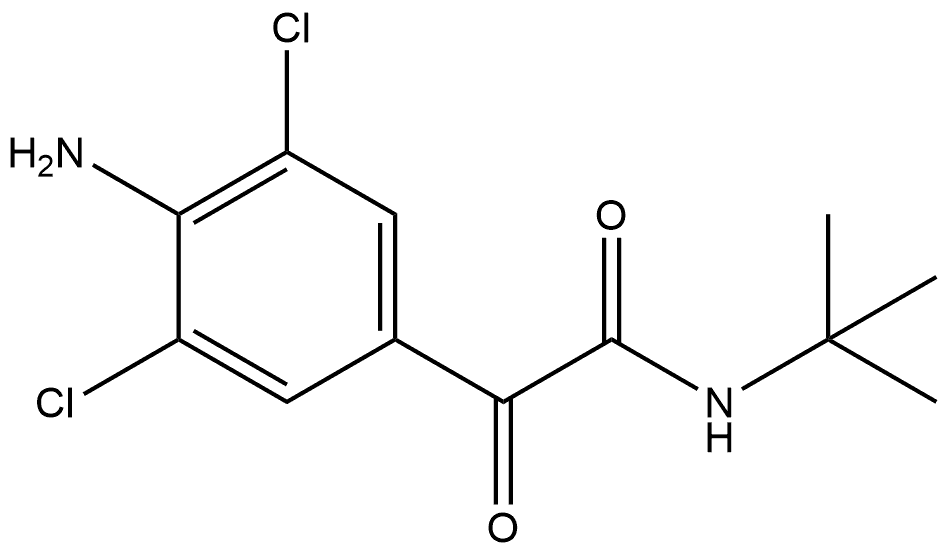
75136-79-7
0 suppliers
inquiry

37148-27-9
101 suppliers
$60.00/2mg
21898-19-1
223 suppliers
$29.00/10mg

37148-27-9
101 suppliers
$60.00/2mg
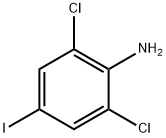
697-89-2
27 suppliers
$55.00/100mg

37148-27-9
101 suppliers
$60.00/2mg
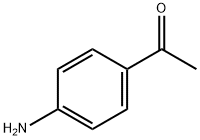
99-92-3
519 suppliers
$6.00/10g

37148-27-9
101 suppliers
$60.00/2mg