
Chloroacetonitrile synthesis
- Product Name:Chloroacetonitrile
- CAS Number:107-14-2
- Molecular formula:C2H2ClN
- Molecular Weight:75.5
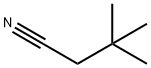
3302-16-7
24 suppliers
inquiry

107-14-2
283 suppliers
$15.00/25g
Yield:107-14-2 89 g
Reaction Conditions:
Stage #1: 3,3-dimethyl-butyronitrilewith chlorine in water at 70;
Stage #2: with acetic acid in water at 50; for 8 h;
Steps:
5.1-5.2 Example 5
:(1) Turn on the exhaust gas absorption system, add 141g of tert-butyl acetonitrile to the reaction flask, then slowly pour chlorine into the four-necked flask containing tert-butyl acetonitrile to react at 70°C, and sample during the reaction Detect to track the reaction process. After the reaction, the mixed solution is directly used in the next step without processing;(2) Add 60g of water and 5g of acetic acid to the four-necked flask, stir, turn on the exhaust gas absorption system, add the mixed liquid after the reaction in step (1) to the water dropwise, and react at 50°C for 8h after the dropwise addition is complete. After the end, the mixed liquid was allowed to stand for stratification, and the lower organic layer was removed and washed with water and rectified to obtain 89 g of colorless liquid chlorocyanomethane.
References:
CN111393325,2020,A Location in patent:Paragraph 0049-0050
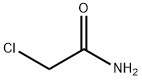
79-07-2
398 suppliers
$5.00/25g

107-14-2
283 suppliers
$15.00/25g

109-74-0
5 suppliers
$20.00/25mL

107-14-2
283 suppliers
$15.00/25g

107-12-0
4 suppliers
$24.69/1ML

107-14-2
283 suppliers
$15.00/25g
75-05-8
988 suppliers
$10.00/10g

107-14-2
283 suppliers
$15.00/25g