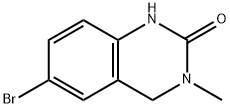
CHEMBRDG-BB 7118966 synthesis
- Product Name:CHEMBRDG-BB 7118966
- CAS Number:328956-24-7
- Molecular formula:C9H9BrN2O
- Molecular Weight:241.08
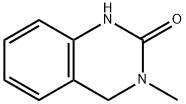
24365-65-9
45 suppliers
$50.00/10g

328956-24-7
24 suppliers
$140.00/100mg
Yield:328956-24-7 84%
Reaction Conditions:
with NBS in dichloromethane at 20; for 2 h;Inert atmosphere;
Steps:
25.1 EXAMPLE 25 Step 1 : Preparation of 6-bromo-3-methyl-3,4-dihydroquinazolin-2(1 /-/)-one,Intermediate 4:
A solution of 3-methyl-3,4-dihydroquinazolin-2(1 H)-one (1 OO.OOg, 61 .655 mmol) in dichloromethane (300 mL) was treated portionwise with N-bromosuccinimide (13.2g, 74.00 mmol, 1 .20 equiv). The red solution was stirred at room temperature under nitrogen for 2 h and a precipitate formed. The orange solid was removed by filtration and the filtrate was washed with water, brine, and dried over Na2SO . This was filtered and concentrated to yield the title compound as a white solid (Yield 84%, 1H NMR (400 MHz, CDCI3) δ 8.20 (br s, 1 H), 7.25 (dd, 1 H, J = 2 Hz, 12 Hz), 7.15 (d, 1 H, J = 2 Hz), 6.62 (d, 1 H, J = 12 Hz), 4.41 (s, 2H), 3.02 (s 3H); LC/MS [M + H]+ = 241 m/z).
References:
WO2013/27168,2013,A1 Location in patent:Page/Page column 46