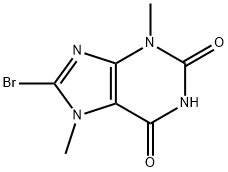
CHEMBRDG-BB 4023079 synthesis
- Product Name:CHEMBRDG-BB 4023079
- CAS Number:15371-15-0
- Molecular formula:C7H7BrN4O2
- Molecular Weight:259.06
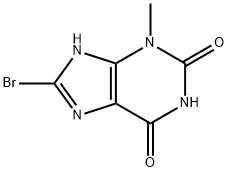
93703-24-3
392 suppliers
$30.00/1g

74-88-4
350 suppliers
$15.00/10g

15371-15-0
15 suppliers
$84.00/250mg
Yield:15371-15-0 93%
Reaction Conditions:
with potassium carbonate in N-methyl-acetamide;water;
Steps:
15 Synthesis of (R)-3,7-Dimethyl-1-(5-hydroxyhexyl)-8-N-methylaminoxanthine (CT12481)
Example 15 Synthesis of (R)-3,7-Dimethyl-1-(5-hydroxyhexyl)-8-N-methylaminoxanthine (CT12481) To a stirring suspension of 8-bromo-3-methylxanthine (prepared as described above for CT12440) (12.25 g, 50.0 mmol) and potassium carbonate (8.62 g, 62.5 mmol) in dimethylformamide (150 ml) was added methyl iodide (7.81 g, 55.0 mmol). After stirring overnight at room temperature, the mixture was poured into ice cold water (400 ml) and stirred at 0-5° C. for 1 hour. The precipitate was filtered, rinsed with water (5*25 ml) and dried under vacuum to provide 8-bromo-3,7-dimethylxanthine (12.10 g, 93% yield) as a beige solid.
References:
US2002/28823,2002,A1
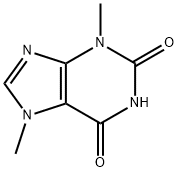
83-67-0
463 suppliers
$5.00/100mg

15371-15-0
15 suppliers
$84.00/250mg
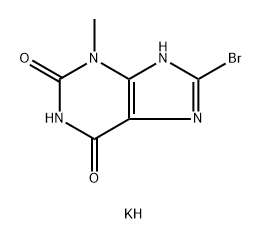
93703-25-4
0 suppliers
inquiry

74-88-4
350 suppliers
$15.00/10g

15371-15-0
15 suppliers
$84.00/250mg
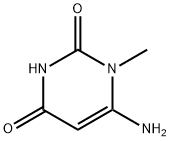
2434-53-9
248 suppliers
$6.00/5g

15371-15-0
15 suppliers
$84.00/250mg

6972-82-3
130 suppliers
$21.00/100mg

15371-15-0
15 suppliers
$84.00/250mg