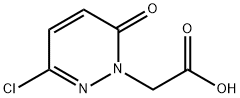
CHEMBRDG-BB 4000145 synthesis
- Product Name:CHEMBRDG-BB 4000145
- CAS Number:89581-61-3
- Molecular formula:C6H5ClN2O3
- Molecular Weight:188.57
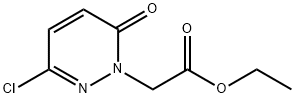
71173-10-9
2 suppliers
inquiry

89581-61-3
21 suppliers
$65.00/100mg
Yield:89581-61-3 2.1 g
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;water at 20; for 6 h;
Steps:
2-1.2 Step 2: Preparation of 2-(3-chloro-6-oxo-pyridazin-l-yl) acetic acid
Step 2: Preparation of 2-(3-chloro-6-oxo-pyridazin-l-yl) acetic acid Ethyl 2-(3-chloro-6-oxo-pyridazin-l-yl)acetate (3.0 g, 13.8 mmol) was hydrolyzed with lithium hydroxide monohydrate (500 mg, 11.9 mmol) in tetrahydrofuran/water (10 mL/5 mL) at room temperature for 6 hours. The resulting mixture was acidified to pH = 2 with aqueous hydrochloric acid (1 M) and extracted with ethyl acetate (30 mL) three times. The combined organic extracts were washed with brine, dried over anhydrous magnesium sulfate, filtered and evaporated under vacuum to give 2-(3-chloro-6-oxo-pyridazin-l-yl)acetic acid (2.1 g) as a solid.
References:
WO2016/23877,2016,A1 Location in patent:Page/Page column 83-84
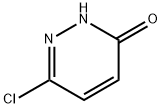
19064-67-6
193 suppliers
$10.00/1g
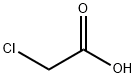
79-11-8
10 suppliers
$27.00/25g

89581-61-3
21 suppliers
$65.00/100mg