Cbz-L-tert-Leucine synthesis
- Product Name:Cbz-L-tert-Leucine
- CAS Number:62965-10-0
- Molecular formula:C14H19NO4
- Molecular Weight:265.3
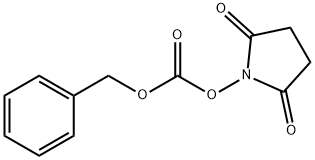
13139-17-8
504 suppliers
$6.00/25g
20859-02-3
604 suppliers
$9.00/5 g

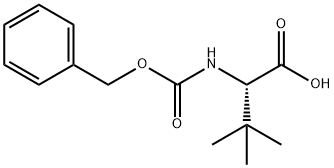
62965-10-0
151 suppliers
$16.00/5g
Yield: 100%
Reaction Conditions:
with triethylamine in methanol at 20; for 14 h;
Steps:
A
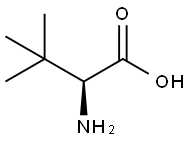
Step A: 2S-Benzyloxycarbonylamino-3,3-dimethyl-butyric acid; To a suspension of L-tert-leucine (11.88 g, 90.7 mmol) in methanol (200 ml) were added triethylamine (26.56 ml, 190 mmol) and N-(benzyloxycarbonyl- oxy)-succinimide (24.88 g, 99.8 mmol). The reaction mixture was stirred at room temperature for 14 h. Methanol was removed in vacuo to afford a viscous pale yellow oil, which was dissolved in ethyl acetate (100 ml). The organic layer was washed with 1M hydrochloric acid (15 ml) and brine, dried over anhydrous magnesium sulfate and filtered. The solvent was removed in vacuo to fumish the title compound as an oil (24 g, quant.). 1H-NMR; δ(CDCl3), 7.43-7.36 (5H, m), 5.36 (1H, d, J = 9.4 Hz), 5.12 (2H, br s), 4.20 (1H, d, J = 9.6 Hz) and 1.02 (9H, s). LRMS: +ve ion 266 [M+H], -ve ion 264 [M-H], 529 [2M-H].
References:
Vernalis (Oxford) Ltd EP1210330, 2005, B1 Location in patent:Page/Page column 9-10

20859-02-3
604 suppliers
$9.00/5 g
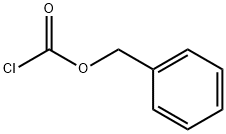
501-53-1
395 suppliers
$10.00/1g

62965-10-0
151 suppliers
$16.00/5g

20859-02-3
604 suppliers
$9.00/5 g
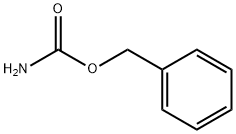
621-84-1
321 suppliers
$7.00/25g

62965-10-0
151 suppliers
$16.00/5g
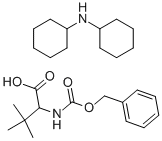
62965-37-1
93 suppliers
$25.89/1G

62965-10-0
151 suppliers
$16.00/5g

20859-02-3
604 suppliers
$9.00/5 g
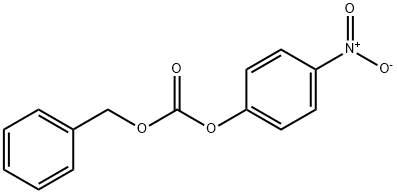
13795-24-9
85 suppliers
$5.00/100mg

62965-10-0
151 suppliers
$16.00/5g