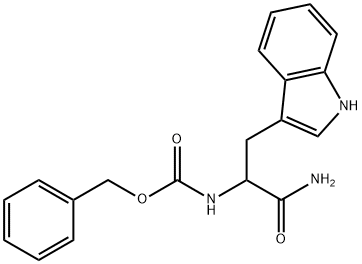
Carbobenzoxy-D,L-tryptophanamide synthesis
- Product Name:Carbobenzoxy-D,L-tryptophanamide
- CAS Number:27018-75-3
- Molecular formula:C19H19N3O3
- Molecular Weight:337.37
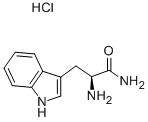
5022-65-1
156 suppliers
$6.00/250mg
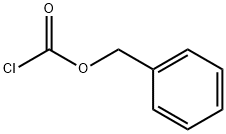
501-53-1
395 suppliers
$10.00/1g

27018-75-3
24 suppliers
inquiry
Yield:27018-75-3 97%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;water at 0; for 1.08333 h;
Steps:
1.a [1-Carbamoyl-2-(3-indolyl)-ethyl]-carbamic acid benzyl ester
To a solution of 8.35 g (0.21 mol) NaOH in 100 ml of water was added 200 ml of THF followed by 25.0 g (0.104 mol) of L-tryptophanamide hydrochloride. The mixture was cooled on ice and 19.6 g (0.114 mol) of benzylchloroformate was added under vigorous stirring during 5 minutes. The ice bath was removed and the mixture was stirred for 1 hour. The mixture was partitioned between 150 ml of water and 250 ml of EtOAC. The organic phase was collected and the aqueous phase extracted with an additional 100 ml of EtOAc. The combined organic phases were washed with water and brine. After evaporation the solid obtained was suspended in 400 ml of diethyl ether and filtered, the sub-title product, 34.0 g (97%), was obtained as a white solid. 1H-NMR (300 MHz, DMSO-d6): δ 2.83-2.97 (1H, m), 3.06-3.16 (1H, m), 3.35 (1H, s), 4.22 (1H, dt, J 9.3 4.2 Hz), 4.95 (2H, s), 6.97 (1H, t, J 6.7 Hz), 7.01-7.17 (3H, m), 7.20-7.37 (6H, m), 7.49 (1H, s), 7.64(1H, d, J 7.9 Hz), 10.81 (1H, s) FAB-MS: m/z 338.1 [MH+]
References:
US6346625,2002,B1 Location in patent:Page column 14