
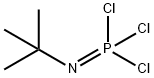
BTPP synthesis
- Product Name:BTPP
- CAS Number:161118-67-8
- Molecular formula:C16H33N4P
- Molecular Weight:312.43
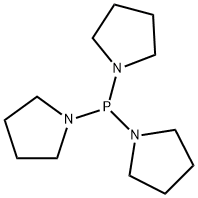
5666-12-6
55 suppliers
$21.00/1g
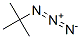
13686-33-4
13 suppliers
inquiry

161118-67-8
50 suppliers
$24.00/250mg
Yield:161118-67-8 64%
Reaction Conditions:
Stage #1:tris-(1-pyrrolidino)phosphine;t-butyl azide at 5 - 85;Inert atmosphere;
Stage #2: with barium(II) oxide at 140; for 40 h;Inert atmosphere;
Steps:
4.3. Synthesis of P1-base
4.3.1. tert-Butylimino-tris(1-pyrrolidinyl)phosphorane.12 R. Schwesinger, J. Willaredt, H. Schlemper, M. Keller, D. Schmitt and H. Fritz, Chem. Ber. 127 (1994), pp. 2435-2454. Full Text via CrossRef12Note: All operations were performed using freshly distilled dry solvents under a positive atmosphere of dry argon. Freshly distilled pyrrolidine (85.20 g, 1.198 mol) and THF (300 mL) were placed into a 1 L three-neck round-bottom flask equipped with a PTFE-coated magnetic stirring bar, thermometer, dropping funnel, and argon inlet and cooled to -40 °C. Then solution of PCl3 (20.05 g, 0.146 mol) in THF (200 mL) was added dropwise at -40 °C. After completion of the addition the reaction mixture was warmed slowly to rt and stirred at ambient temperature for 18 h. The solvent was removed from the reaction flask in vacuo (rt, 50 mbar) and the solid residue was washed twice with hexane (2×100 mL). The combined hexane fractions were concentrated in vacuo (rt, 50 mbar) to afford tris(1-pyrrolidinyl)phosphine (29.34 g, 0.122 mol) as colorless liquid. The obtained phosphine was placed into a 250 mL three-necked round-bottom flask equipped with a PTFE-coated magnetic stirring bar, thermometer, dropping funnel, and argon inlet, cooled to 5 °C and tert-butyl azide19 (14.5 g, 0.146 mol) was added dropwise. The reaction mixture was stirred overnight at rt, heated at 85 °C for 1 h and cooled to rt. Volatiles were removed at 50 mbar for 30 min. BaO (1.0 g) was added and the reaction mixture was heated at 140 °C for 40 h. Vacuum distillation over BaO afforded the P1-base (29.1 g, 64%) as a viscous colorless oil; bp 112-114 °C (0.15 mbar) [lit.12 bp 108 °C (0.05 Torr)]; 1H NMR (C6D6) δ 3.08-3.12 (m, 12H, α-H-pyrrolidinyl), 1.60-1.50 (m, 12H, β-H-pyrrolidinyl), 1.56 (s, 9H, t-Bu); 31P NMR (C6D6) δ 8.8.
References:
Boltukhina, Ekaterina V.;Sheshenev, Andrey E.;Lyapkalo, Ilya M. [Tetrahedron,2011,vol. 67,# 30,p. 5382 - 5388] Location in patent:experimental part

123-75-1
543 suppliers
$10.00/5g
18854-80-3
3 suppliers
inquiry

161118-67-8
50 suppliers
$24.00/250mg

5666-12-6
55 suppliers
$21.00/1g

161118-67-8
50 suppliers
$24.00/250mg