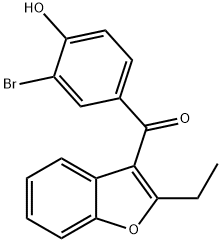
bromobenzarone synthesis
- Product Name:bromobenzarone
- CAS Number:94729-09-6
- Molecular formula:C17H13BrO3
- Molecular Weight:345.19
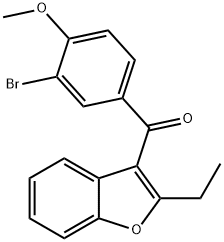
1352799-79-1
3 suppliers
inquiry

94729-09-6
36 suppliers
inquiry
Yield: 57%
Reaction Conditions:
with sodium thioethylate in N,N-dimethyl-formamide at 105 - 110; for 16 h;
Steps:
In a RBF/SB, 56 (0.05 g, 0.139 mmol) was weighed out. The material was diluted with DMF (2.5 mL), NaSEt (0.058 g, 0.696 mmol) was added and warmed (105-1 10 °C; 16 h). The mixture was then quenched with 2 vol NH4C1 aq and extracted with EtOAc (4 x 20 mL). The organic phase was dried (Na2S04), filtered, concentrated under reduced pressure and purified via Si02 chromatography (3: 1 ; Hex: EtOAc) to give 61 (23.2 mg, 0.079 mmol, 57% yield) as a light yellow oil. 1H-NMR (400 MHz) CDC13: 8.06-8.05 (d, 1H), 7.76-7.74 (dd, 1H), 7.50- 7.48 (d, 1H), 7.41-7.39 (d, 1H), 7.31-7.29 (t, 1H), 7.23-7.19 (t, 1H), 7.10-7.08 (d, 1H), 6.21 (bs, 1H), 2.94-2.89 (q, 2H), 1.37-1.33 (t, 3H); 13C-NMR (100 MHz) CDC13: 189.2, 166.0, 156.3, 153.7, 133.8, 133.2, 131.1 , 126.8, 124.5, 123.6, 121.1 , 1 15.9, 1 15.8, 1 1 1.1 , 1 10.5, 21.8, 12.3. LC/MS-MS: 344.9 -? 198.8 m/z GS1 and GS2 at 25, DP = 61, CE = 33, CXP = 12, tR = 4.59 min.
References:
J-PHARMA CO., LTD.;WEMPE, Michael, F.;ENDOU, Hitoshi WO2012/48058, 2012, A2 Location in patent:Page/Page column 33-34
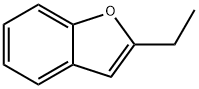
3131-63-3
173 suppliers
$10.00/5g

94729-09-6
36 suppliers
inquiry
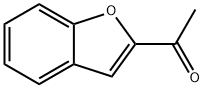
1646-26-0
139 suppliers
$8.00/1g

94729-09-6
36 suppliers
inquiry
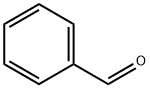
100-52-7
949 suppliers
$17.30/2g

94729-09-6
36 suppliers
inquiry
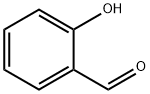
90-02-8
455 suppliers
$9.00/1g

94729-09-6
36 suppliers
inquiry