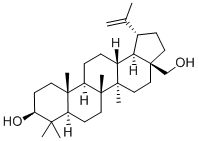
Betulin synthesis
- Product Name:Betulin
- CAS Number:473-98-3
- Molecular formula:C30H50O2
- Molecular Weight:442.72
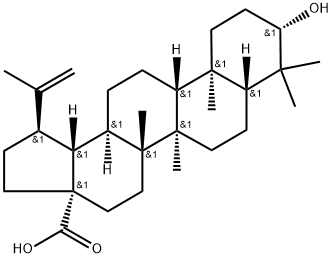
472-15-1
422 suppliers
$5.00/25mg

473-98-3
398 suppliers
$16.35/10mg
Yield:473-98-3 90%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran for 5 h;Reflux;Inert atmosphere;Sealed tube;
Steps:
4.4. Reduction of C-28 carboxylic acid group of 1, 8, and 9
General procedure: To a dried 25 mL glass vial equipped with a magnetic stirrer, containing13.7 mg (0.03 mmol) of (+)-ursolic acid (1), 2 mL of a 0.02Msolution of LiAlH4 in dried THF (0.04 mmol) were added to the vialunder argon. The vial was sealed, and the mixture was stirred withreflux for 5 h, it was cooled to room temperature. Next, the reactionmixture was diluted with aqueous ether, extracted with CH2Cl2 (DCM),dried over anhydrous Na2SO4, evaporated to dryness and chromatographedover silica gel to obtain 12.0 mg (0.027 mmol) of the product,(+)-uvaol (10), with a reaction yield of 90.9%. Following this sameprocedure, 12.0 mg (0.03 mmol) of the product (+)-erythrodiol (11,with a yield of 90.9%) were prepared from 13.7 mg (0.03 mmol) of(+)-oleanolic acid (8, purchased from Sigma), and 7.9 mg(0.018 mmol) of the product (+)-betulin (12) (with a yield of 90.0%)were prepared from 9.1 mg (0.02 mmol) of (+)-betulinic acid (9, isolatedfrom our previous study).
References:
Ren, Yulin;Anaya-Eugenio, Gerardo D.;Czarnecki, Austin A.;Ninh, Tran Ngoc;Yuan, Chunhua;Chai, Hee-Byung;Soejarto, Djaja D.;Burdette, Joanna E.;de Blanco, Esperanza J. Carcache;Kinghorn, A. Douglas [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 15,p. 4452 - 4460] Location in patent:supporting information
1721-69-3
51 suppliers
$58.00/10 mg

473-98-3
398 suppliers
$16.35/10mg

1721-69-3
51 suppliers
$58.00/10 mg

473-98-3
398 suppliers
$16.35/10mg
27570-20-3
5 suppliers
inquiry