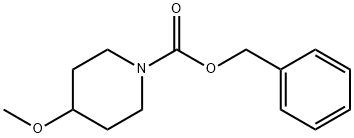
BENZYL 4-METHOXYPIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:BENZYL 4-METHOXYPIPERIDINE-1-CARBOXYLATE
- CAS Number:553672-12-1
- Molecular formula:C14H19NO3
- Molecular Weight:249.31
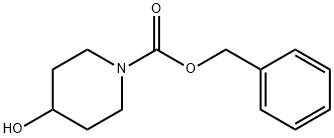
95798-23-5
139 suppliers
$5.00/250mg

74-88-4
349 suppliers
$15.00/10g

553672-12-1
14 suppliers
inquiry
Yield:553672-12-1 94%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 0 - 20; for 16.5 h;
Steps:
39 Production Example 39; benzyl 4-methoxy-1-piperidinecarboxylate
Production Example 39 benzyl 4-methoxy-1-piperidinecarboxylate To a 20 ml tetrahydrofuran solution of 1.87 g (7.95 mmol) of benzyl 4-hydroxy-1-piperidinecarboxylate, 413 mg (60% in oil, 10.33 mmol) of sodium hydride and 792 μl (12.72 mmol) of methyl iodide were added at 0°C, and the mixture was stirred at room temperature for 16.5 hours.. Then, the reaction mixture was diluted with ethyl acetate, and washed with water.. The washed system was dried over sodium sulfate, and then the solvent was distilled off under reduced pressure.. The residue was purified by silica gel column chromatography (hexane/ethyl acetate = 2/1) to obtain 2.08 g (94%) of the captioned compound.
References:
EP1454897,2004,A1 Location in patent:Page 26

74-88-4
349 suppliers
$15.00/10g

553672-12-1
14 suppliers
inquiry
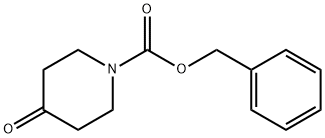
19099-93-5
318 suppliers
$5.00/1g

553672-12-1
14 suppliers
inquiry