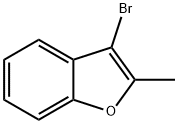
BENZOFURAN, 3-BROMO-2-METHYL- synthesis
- Product Name:BENZOFURAN, 3-BROMO-2-METHYL-
- CAS Number:58863-48-2
- Molecular formula:C9H7BrO
- Molecular Weight:211.06
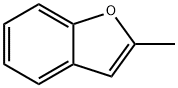
4265-25-2
141 suppliers
$23.00/100mg

58863-48-2
30 suppliers
$99.00/100mg
Yield:58863-48-2 56%
Reaction Conditions:
with N-Bromosuccinimide in tetrahydrofuran at 5;
Steps:
Synthesis of Compound 16-f
A solution of 16-g (2.89 g, 21.91 mmol) dissolved in tetrahydroffiran (80 mE) was cooled to 5° C., and then N135 (4.68 g, 26.29 mmol) was slowly added thereto. The reactionsolution was reacted at normal temperature overnight, and then poured into sodium thiosulfate solution, and extracted with ethyl acetate (80 mLx3). The combined organic phase was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluted with petroleum ether) to obtain compound 16-f (2.57 g, yield 56%) as a colourless liquid. ‘H NMR (400 MHz, CDC13-MeOD): ? 7.37-7.3 1 (m, 2H), 7.20-7.18 (m, 2H), 2.40 (s, 3H).
References:
US2016/214994,2016,A1 Location in patent:Paragraph 0151; 0152
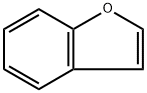
271-89-6
258 suppliers
$9.00/1g

58863-48-2
30 suppliers
$99.00/100mg