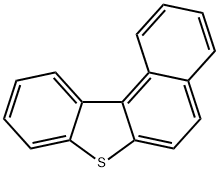
BENZO(B)NAPHTHO(1,2-D)THIOPHENE synthesis
- Product Name:BENZO(B)NAPHTHO(1,2-D)THIOPHENE
- CAS Number:205-43-6
- Molecular formula:C16H10S
- Molecular Weight:234.32
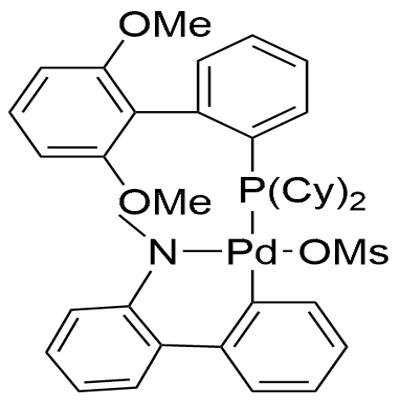
1599466-87-1
85 suppliers
$35.00/100mg

205-43-6
121 suppliers
$18.00/100mg
Yield:205-43-6 53%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);copper diacetate;sodium pivalate in N,N-dimethyl-formamide at 150; for 5 h;Inert atmosphere;
Steps:
General procedure for the preparation of dibenzothiophenes form the 2-iodinated diphenylthioethers using Pd3(dba)2:
General procedure: Under argon flush, a flask was charged with the substrates of diaryl thioethers (0.58 mmol), DMF (10 mL), Pd3(dba)2 (105 mg, 0.115 mmol), Cu(OAc)2 (23 mg, 0.115 mmol) amd sodium pivalate hydrate (72 mg, 0.58 mmol), and then the mixture was maintained at 150°C with stirring for 5 h under argon atmosphere. After completion by TLC analysis, the reaction mixture was diluted with EtOAc and water. The aqueous phase was extracted with EtOAc twice and the combined organic phases were washed wtih brine and dried over MgSO4. Then solvent was evaporated under vacuum and the crude product was purified by column chromatography on silica gel. eluting with a mixture of petroleum ether and EtPAc to give dibenzothiophenes. Yields are listed in Table 2.
References:
Chen, Ya-Fang;Ma, Rui-Yue;Zhou, Lu-Nan;Du, Zhenting;Zhang, Tao [Heterocycles,2016,vol. 92,# 10,p. 1874 - 1881]
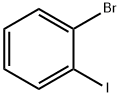
583-55-1
468 suppliers
$7.00/5g
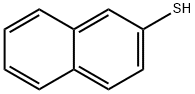
91-60-1
219 suppliers
$15.00/1g
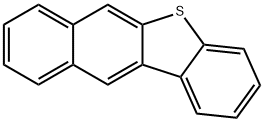
243-46-9
22 suppliers
$218.00/10mg

205-43-6
121 suppliers
$18.00/100mg
![Propanoic acid, 3-[[1-(2-fluorophenyl)-2-naphthalenyl]thio]-, ethyl ester](/CAS/20210305/GIF/1264712-19-7.gif)
1264712-19-7
0 suppliers
inquiry

205-43-6
121 suppliers
$18.00/100mg
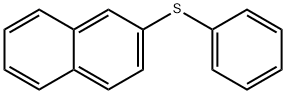
7570-96-9
1 suppliers
inquiry

205-43-6
121 suppliers
$18.00/100mg
![2-IODO-BENZO[B]THIOPHENE](/CAS/GIF/36748-89-7.gif)
36748-89-7
40 suppliers
$28.00/100mg
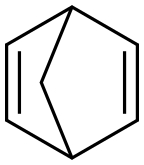
121-46-0
154 suppliers
$30.00/10g
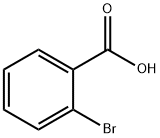
88-65-3
340 suppliers
$5.00/5g

205-43-6
121 suppliers
$18.00/100mg