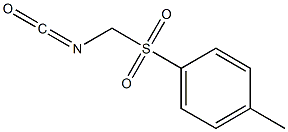
Benzene, 1-[(isocyanatomethyl)sulfonyl]-4-methyl- synthesis
- Product Name:Benzene, 1-[(isocyanatomethyl)sulfonyl]-4-methyl-
- CAS Number:10564-55-3
- Molecular formula:C9H9NO3S
- Molecular Weight:211.2377
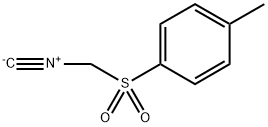
36635-61-7
504 suppliers
$5.00/5g
![Benzene, 1-[(isocyanatomethyl)sulfonyl]-4-methyl-](/CAS/20180601/GIF/10564-55-3.gif)
10564-55-3
0 suppliers
inquiry
Yield:10564-55-3 53%
Reaction Conditions:
with dimethyl sulfoxide;trifluoroacetic anhydride in dichloromethane at -78 - 20; for 0.166667 h;Inert atmosphere;
Steps:
Preparation of Tosylmethylisocyanate 2
A solution of tosylmethylisonitrile (1.95 g, 10 mmol) in dry CH2Cl2 (30 mL) and dryDMSO (710 μL, 10 mmol) under N2 in a 100 mL RBF was cooled to -78 oC, then TFAA (840μL, 6.0 mmol) was added. The resulting solution was stirred vigorously at -78 oC for 5 min, then warmed to rt and stirred for 5 min. One drop of the reaction solution was removed via Pasteur pipet and submitted to IR analysis to confirm that the reaction was complete. Solvent was then removed using a rotary evaporator and the residue was concentrated in vacuo (0.2 Torr, 5 min) to afford tosylmethylisocyanate (2.11 g, 100%) as a yellow oil. The crude oil was dissolved indiethyl ether (14 mL), and the resulting clear solution was transferred to a clean 20 mL glassvial. Hexanes (6 mL) was added, and the vial was capped and shaken. The resulting suspensionwas transferred to another clean 20 mL glass vial, which was then left untouched in a fridge to crystallize (-20 oC, 2 h, see Note). The supernatant was decanted and the newly formed crystalswere concentrated in vacuo (0.2 Torr, 30 min) to remove traces of solvent, affordingtosylmethylisocyanate 2 (1.12 g, 53%) as pale yellow crystals: mp (69-70 oC), 1H NMR, and IRmatched literature values.1 1H NMR (300 MHz, CDCl3) δ 7.84 (d, J=8.2 Hz, 2H), 7.42 (d, J=8.2Hz, 2H), 4.39 (s, 2H), 2.49 (s, 3H). IR (neat) 2999 (m), 2934 (m), 2246 (s), 1595 (m).
References:
Le, Hoang V.;Ganem, Bruce [Tetrahedron Letters,2014,vol. 55,# 12,p. 2003 - 2005] Location in patent:supporting information
![Carbamic acid, thio[(p-tolylsulfonyl)methyl]-, S-ethyl ester (8CI)](/CAS/20240320/GIF/28126-30-9.gif)
28126-30-9
0 suppliers
inquiry
![Benzene, 1-[(isocyanatomethyl)sulfonyl]-4-methyl-](/CAS/20180601/GIF/10564-55-3.gif)
10564-55-3
0 suppliers
inquiry