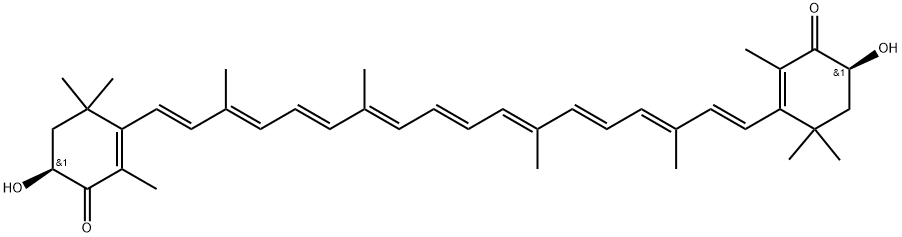
Astaxanthin synthesis
- Product Name:Astaxanthin
- CAS Number:472-61-7
- Molecular formula:C40H52O4
- Molecular Weight:596.85
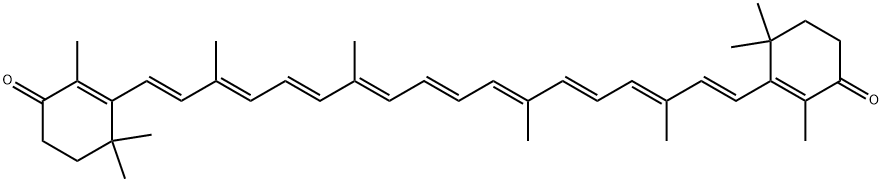
514-78-3
238 suppliers
$90.00/5G

472-61-7
604 suppliers
$8.00/10mg
Yield:472-61-7 4.48 g
Reaction Conditions:
Stage #1: canthaxanthinwith iodosylbenzene;sodium hydroxide in methanol at 10 - 20; for 12.5 h;Inert atmosphere;
Stage #2: with sulfuric acid in water at 20; for 5 h;Reagent/catalyst;Temperature;Solvent;
Steps:
1-4
Under nitrogen protection,Put 5.64g (10mmol) of canthaxanthin in a 500ml three-necked flask, add 180ml methanol to dissolve it,Then add 3.30g (15mmol) of oxidant iodosylbenzene,Lower the reaction temperature to 10°C, stir and mix well,Dissolve 0.20g (5.0mmol) of the catalyst sodium hydroxide in 20ml methanol, within 30 minutes,Slowly add it dropwise to the reaction system. After the dropwise addition is completed, the temperature is raised to room temperature and reacted under normal pressure for 12hAfter the reaction, methanol was removed under reduced pressure,Add water and dichloromethane for washing and liquid separation,An organic phase containing the crude dialkoxy ketal is obtained.Transfer the above-mentioned organic phase to a 500ml three-necked flask, add 1.96g (1.0mmol) of 5wt% sulfuric acid aqueous solution, and stir the reaction at room temperature. After reacting for 5h, add 2.0mmol sodium bicarbonate and 5wt% aqueous solution for quenching For the reaction, the organic phase was washed 3 times with water, the organic phase was separated, dried with anhydrous magnesium sulfate, concentrated, and then recrystallized with acetone to obtain 4.48 g of purple astaxanthin with a yield of 75%.
References:
CN111747876,2020,A Location in patent:Paragraph 0042-0054
![2,4,6,8,10,12-Tridecahexaenal, 13-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexen-1-yl]-2,7,11-trimethyl-, (2E,4E,6E,8E,10E,12E)-](/CAS/20210305/GIF/72523-68-3.gif)
72523-68-3
0 suppliers
inquiry

472-61-7
604 suppliers
$8.00/10mg
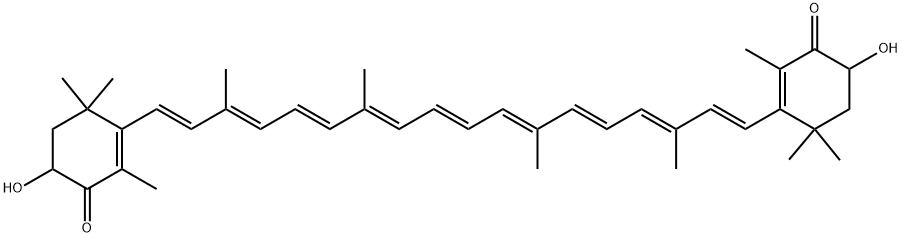
7542-45-2
67 suppliers
$52.00/5mg

472-61-7
604 suppliers
$8.00/10mg
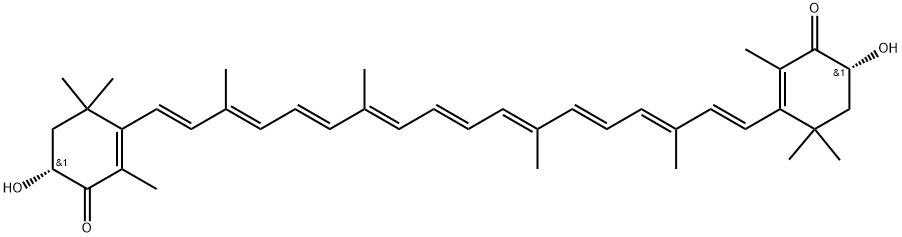
60760-95-4
1 suppliers
inquiry
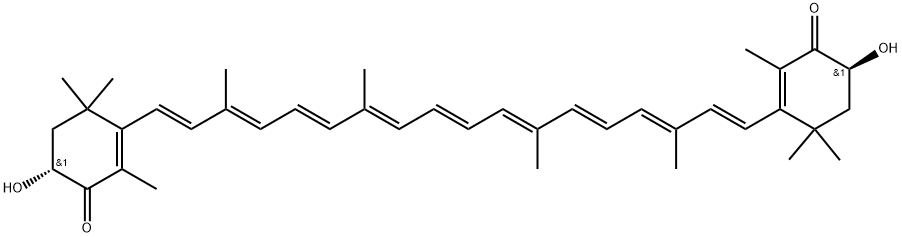
71772-51-5
3 suppliers
inquiry