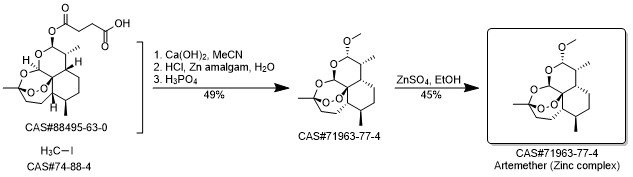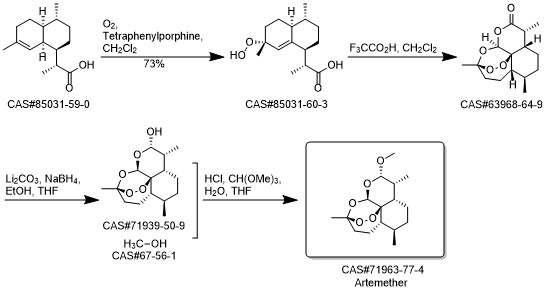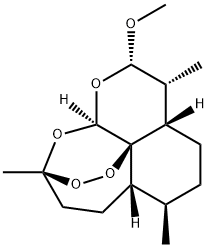
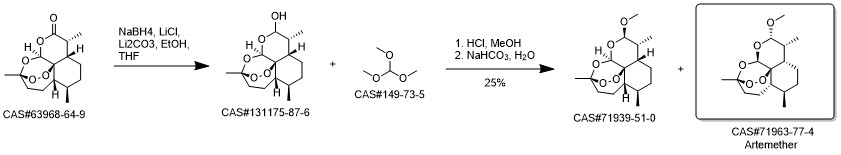
Artemether synthesis
- Product Name:Artemether
- CAS Number:71963-77-4
- Molecular formula:C16H26O5
- Molecular Weight:298.37
Continuous synthesis of artemisinin-derived medicines; Gilmore, Kerry; Kopetzki, Daniel; Lee, Ju Weon; Horvath, Zoltan; McQuade, D. Tyler; Seidel-Morgenstern, Andreas; Seeberger, Peter H. Chemical Communications (Cambridge, United Kingdom); Volume 50; Issue 84; Pages 12652-12655; Journal; 2014

67-56-1
752 suppliers
$9.00/25ml
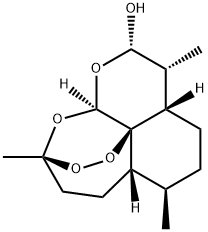
71939-50-9
386 suppliers
$5.00/50mg

71963-77-4
567 suppliers
$29.00/1g
Yield:71963-77-4 756 g
Reaction Conditions:
with perchloric acid in dichloromethane at 30 - 38; for 0.166667 h;Large scale;Temperature;
Steps:
1 Example 1
1. examines lost to view complete preparation equipment, equipment clean, ready to complete the original accessories, including the original supplementary product quality detection qualified, filtering, such as the confirmation number.
2. In the reaction, first add dichloromethane 12.8L. Heat to 30 degrees. Afterwards, add dihydroartemisinin 1 kg. After heating under stirring to 38 degrees. Add methanol 4.2L. At the same time, add perchloric acid 200 ml,. At 38 °C, react for 10 minutes; for when the thin chromatographic analysis detection reaction to the end of the, adding anhydrous carbonate sodium accent 0.35K, adjusting PH=7, the termination of the reaction, the reaction liquid filter, to be concentrated.
3. concentrated by filtration of the reaction solution in the 55 °C the left and right water bath concentration to see white the crystal rests stop after precipitation concentrated.
4. crystallization by concentration of the mother liquor after freezing crystallization, centrifugal filtration, add 2L deionized water filtering, to obtain the coarse grain.
5. the refined crystal is added in the 2L of 0.09, stirring 5 minutes, adding 10L60 - 90-range petroleum ether extraction, 60 degrees concentrated to white crystals, tube to saturated solution cooling crystallization, centrifugal filtration, then adding 2L deionized water filtering, high-performance liquid detecting purity of 99.1%.
6. drying and vacuum pressure reducing water bath drying, temperature 50 °C, 120 minutes, high efficiency liquid phase sample detection, the purity is 98.9%, weighing 756g.
7. packaging adopts a double-layer aluminum foil material packing, sealing, completing the label (name, production lot number, date of production and the like), the low-temperature storage.
References:
CN106565738,2017,A Location in patent:Paragraph 0012-0036

71939-50-9
386 suppliers
$5.00/50mg
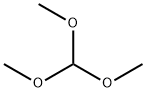
149-73-5
530 suppliers
$12.00/5g

71963-77-4
567 suppliers
$29.00/1g
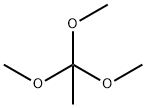
1445-45-0
341 suppliers
$10.00/5mL

71939-50-9
386 suppliers
$5.00/50mg

71963-77-4
567 suppliers
$29.00/1g
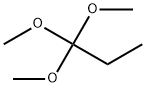
24823-81-2
194 suppliers
$14.00/5g

71939-50-9
386 suppliers
$5.00/50mg

71963-77-4
567 suppliers
$29.00/1g

67-56-1
752 suppliers
$9.00/25ml
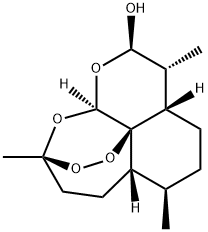
81496-81-3
119 suppliers
inquiry

71963-77-4
567 suppliers
$29.00/1g