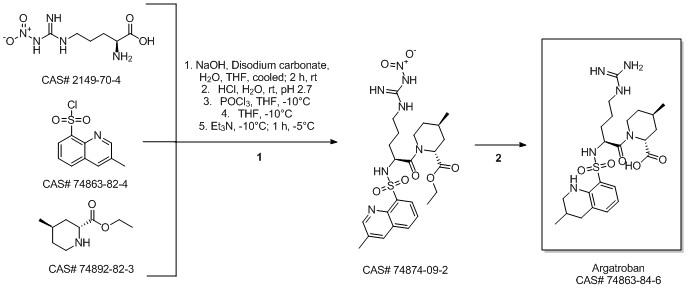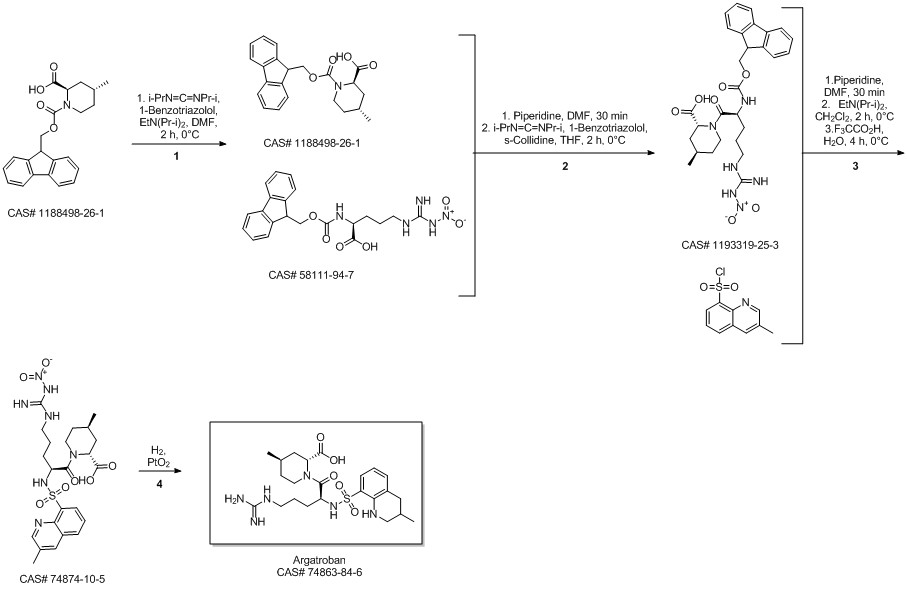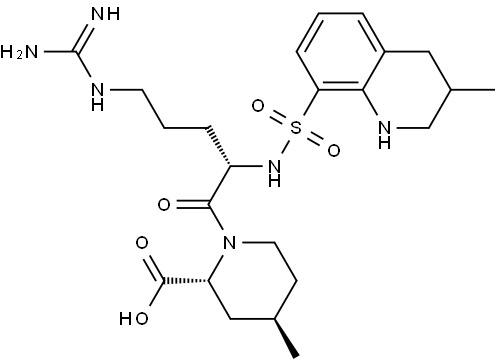
Argatroban synthesis
- Product Name:Argatroban
- CAS Number:74863-84-6
- Molecular formula:C23H36N6O5S
- Molecular Weight:508.64
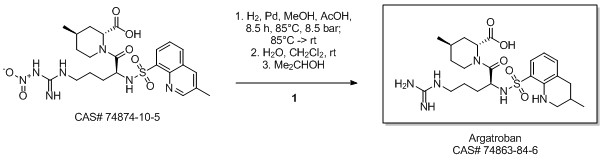
Libralon, Giovanna; Nicole, Andrea; Zanon, Jacopo. Method for the preparation of argatroban monohydrate. Assignee Lundbeck Pharmaceuticals Italy S.p.A.. 2008.
![2-PIPERIDINECARBOXYLIC ACID, 1-[5-[IMINO(NITROAMINO)METHYL]AMINO]-2-[[(3-METHYL-8-QUINOLINYL)SULFONYL]AMINO]-1-OXOPENTYL]-4-METHYL-,[2R-[1(S*), 2ALPHA, 4BETA]]-](/CAS/GIF/74874-10-5.gif)
74874-10-5
118 suppliers
inquiry

74863-84-6
287 suppliers
$32.00/5mg
Yield:74863-84-6 93%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in methanol;acetic acid at 85; under 6375.64 Torr; for 8 h;Product distribution / selectivity;
Steps:
3
Example 2: treatment of the crude argatroban with NaOH and crystallization from normal propanolA one-litre glass autoclave was fed with 50 g of (2f?,4/:?)-1 -[NG-nitro-N2-(3-methyl- 8-quinolinesulphonyl)-L-arginyl]-4-methyl-2-piperidine carboxylic acid, 375 ml of
References:
WO2009/124906,2009,A2 Location in patent:Page/Page column 20-21
![2-Piperidinecarboxylic acid, 1-[(2S)-5-[[imino(nitroamino)methyl]amino]-2-[[(3-methyl-8-quinolinyl)sulfonyl]amino]-1-oxopentyl]-4-methyl-, phenylmethyl ester, (2R,4R)-](/CAS/20200611/GIF/367952-84-9.gif)
367952-84-9
0 suppliers
inquiry

74863-84-6
287 suppliers
$32.00/5mg
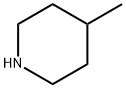
626-58-4
203 suppliers
$10.00/1g

74863-84-6
287 suppliers
$32.00/5mg

123387-50-8
61 suppliers
$65.00/500mg

74863-84-6
287 suppliers
$32.00/5mg
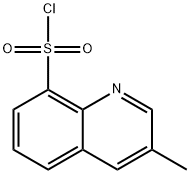
74863-82-4
233 suppliers
$15.00/250mg

74863-84-6
287 suppliers
$32.00/5mg