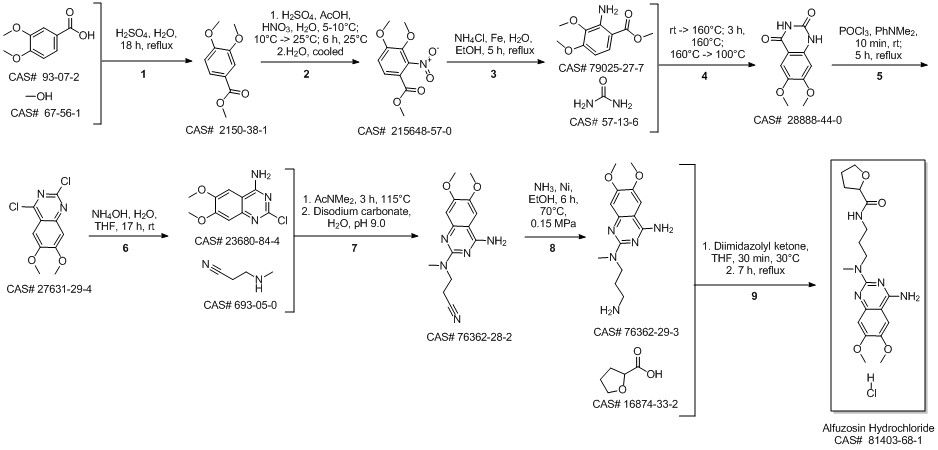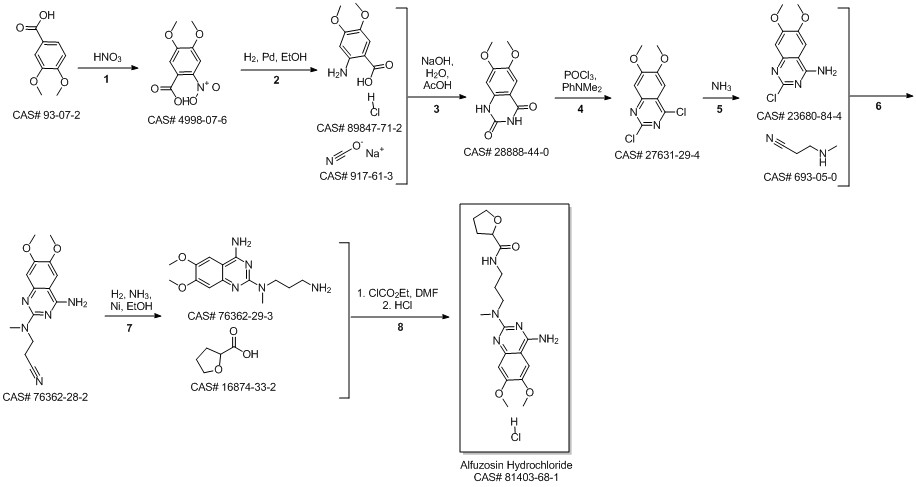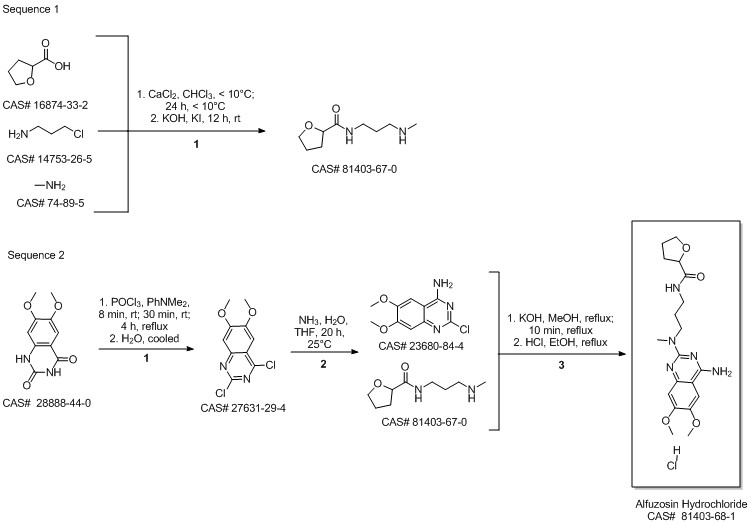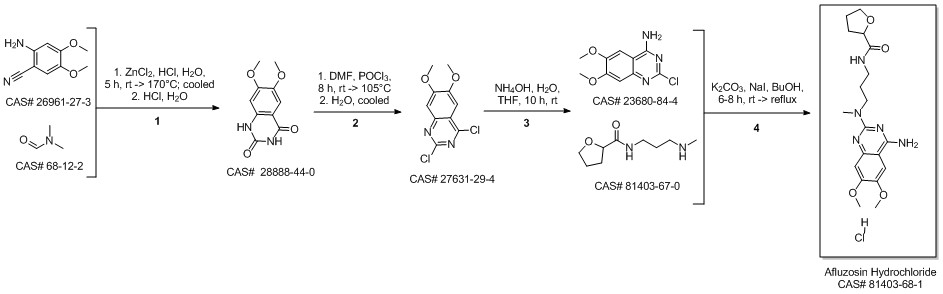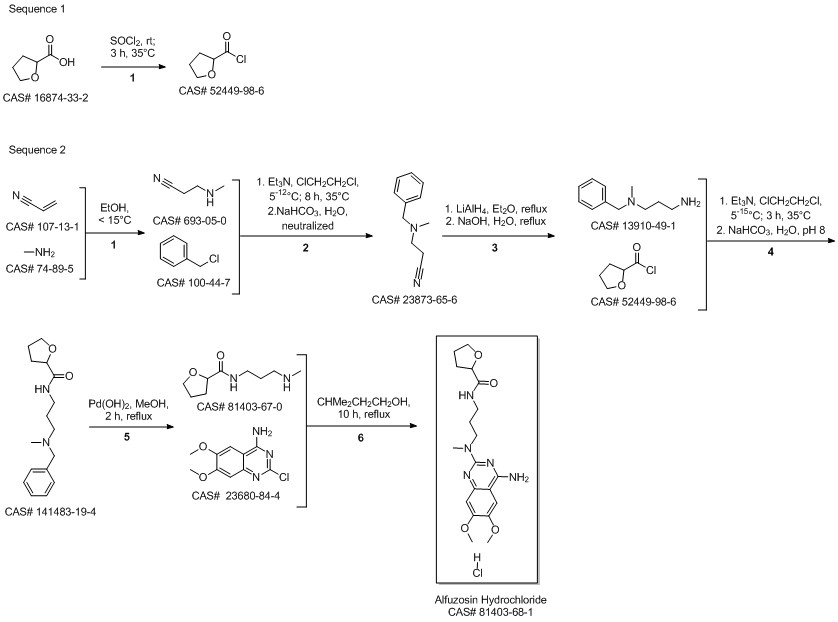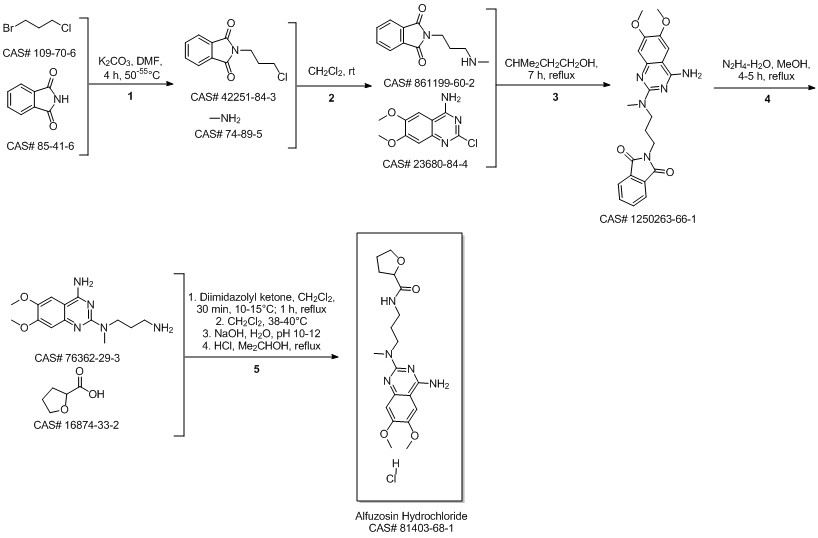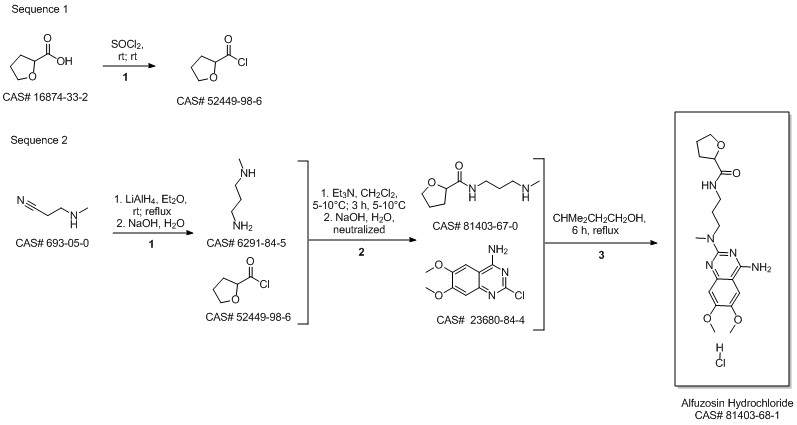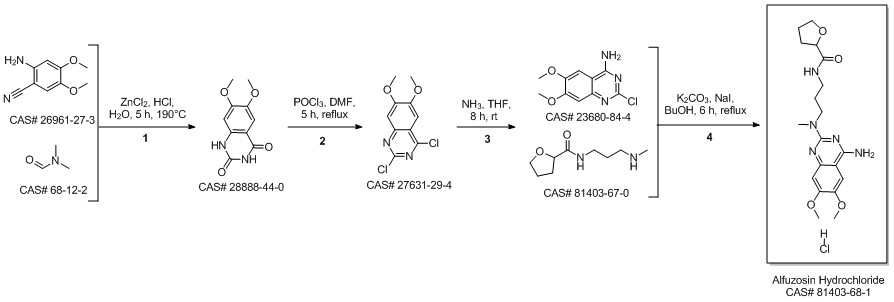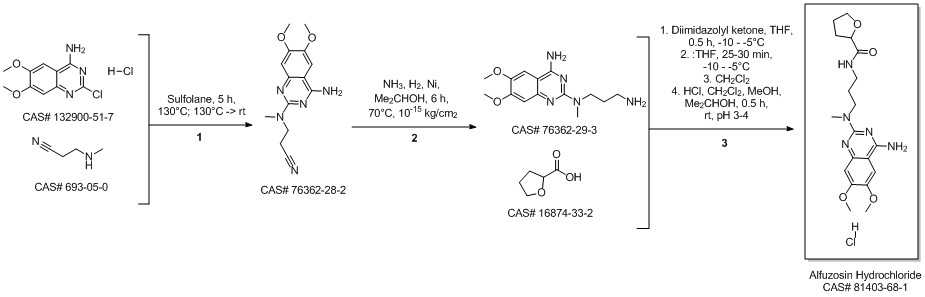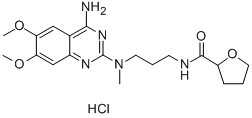
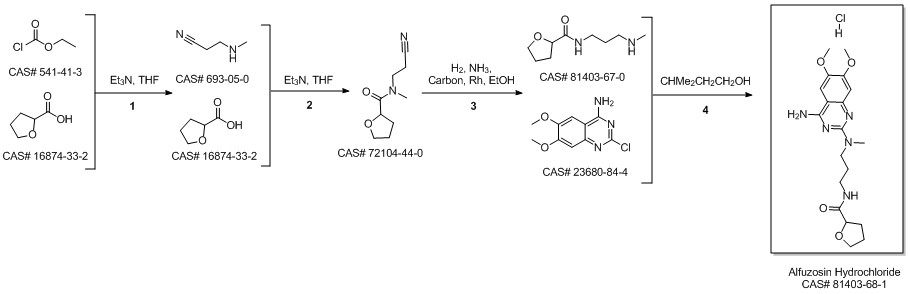
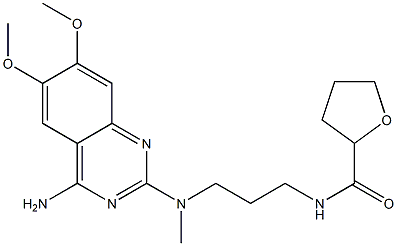
Alfuzosin hydrochloride synthesis
- Product Name:Alfuzosin hydrochloride
- CAS Number:81403-68-1
- Molecular formula:C19H28ClN5O4
- Molecular Weight:425.91
Manoury, Philippe M.; Binet, Jean L.; Dumas, Andre P.; Lefevre-Borg, Francoise; Cavero, Icilio. Synthesis and antihypertensive activity of a series of 4-amino-6,7-dimethoxyquinazoline derivatives. Journal of Medicinal Chemistry. Volume 29. Issue 1. Pages 19-25. 1986.
81403-80-7
131 suppliers
inquiry

81403-68-1
370 suppliers
$28.00/25mg
Yield:81403-68-1 88%
Reaction Conditions:
with hydrogenchloride in ethanol at 20 - 25;
Steps:
4
Example 4:Preparation of anhydrous Alfuzosin hydrochloride: Alfuzosin base (5g, 0.013mol) is charged to a flask and ethanol (110ml) added. The mixture is refluxed for 15 min. and the solid dissolved. Activated charcoal is added to this solution and the suspension is stirred for lOmin., filtered and washed with 5ml hot ethanol. The filtrate is cooled down to 20-250C and the ethanol saturated by hydrogen chloride gas (1.5ml) is added. Then diethyl ether (25ml) and water (0.09ml) were slowly added to the mixture. The mixture is then stirred at room temperature for 20 hours. The mixture is then cooled
References:
UNICHEM LABORATORIES LIMITED WO2008/84493, 2008, A2 Location in patent:Page/Page column 9-10
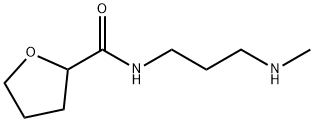
81403-67-0
53 suppliers
inquiry
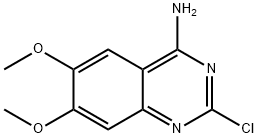
23680-84-4
466 suppliers
$8.00/5g

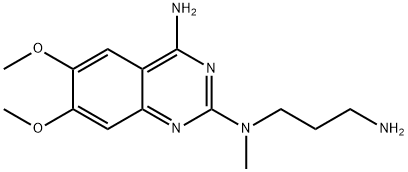
81403-68-1
370 suppliers
$28.00/25mg
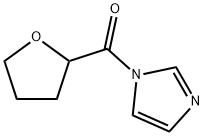
76362-29-3
30 suppliers
inquiry
1005197-73-8
1 suppliers
inquiry

81403-68-1
370 suppliers
$28.00/25mg
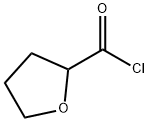
52449-98-6
43 suppliers
$33.00/100mg

76362-29-3
30 suppliers
inquiry

81403-68-1
370 suppliers
$28.00/25mg
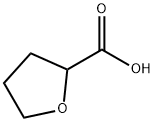
16874-33-2
292 suppliers
$6.00/5g

81403-68-1
370 suppliers
$28.00/25mg