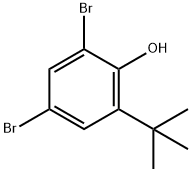
AKOS BBS-00008108 synthesis
- Product Name:AKOS BBS-00008108
- CAS Number:15460-12-5
- Molecular formula:C10H12Br2O
- Molecular Weight:308.01
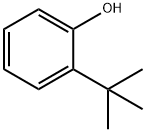
88-18-6
173 suppliers
$16.00/25mL

15460-12-5
21 suppliers
$186.00/1g
Yield:15460-12-5 76%
Reaction Conditions:
with N-Bromosuccinimide;triphenylphosphine sulfide in dichloromethane at 20; for 12 h;
Steps:
I. General Halogenation Procedure of Phenols
General procedure: To phenol (1.0 equiv) was added NXS (1.0 or 2.0 equiv, X =Cl, Br), Lewis Base catalyst (2% to5%) in .1M DCM. The reaction was stirred at room temperature for 12 hours. The mixture wasthen washed three times with NaHCO3. The organic layer was collected and the aqueous layerwas extracted three times with DCM. The organic layers were combined, dried with Na2SO4 andconcentrated in vacuo to yield crude products. Purification through normal phase flash columnchromatography (hexanes:EtOAc::100:0 to 95:5) afforded the desired product. Selectivity ofortho-monohalogenated phenols was given by 1,1’-((1R,2R)-cyclohexane-1,2-diyl)bis(3-(3,5-bis(trifluoromethyl)phenyl) thiourea) (Nagasawa’s Thiourea) while selectivity of paramonohalogenatedphenols was given by 2,2’-Bis(diphenyl-phosphino)-1,1’-binaphthyl (BINAP) disulfide catalyst. Triphenylphosphine sulfide catalyst was used for disubstituted halogenations.The catalyst selection is in agreeance with the literature1.
References:
Dinh, Andrew N.;Noorbehesht, Ryan R.;Toenjes, Sean T.;Jackson, Amy C.;Saputra, Mirza A.;Maddox, Sean M.;Gustafson, Jeffrey L. [Synlett,2018,vol. 29,# 16,p. 2155 - 2160] Location in patent:supporting information

88-18-6
173 suppliers
$16.00/25mL
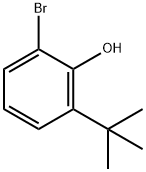
23159-87-7
32 suppliers
inquiry

15460-12-5
21 suppliers
$186.00/1g
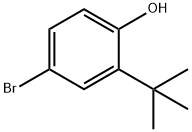
10323-39-4
116 suppliers
$110.00/1g

23159-87-7
32 suppliers
inquiry

15460-12-5
21 suppliers
$186.00/1g
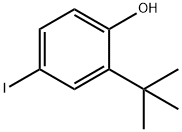
60803-25-0
5 suppliers
inquiry

15460-12-5
21 suppliers
$186.00/1g
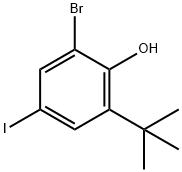
60803-27-2
6 suppliers
inquiry

15460-12-5
21 suppliers
$186.00/1g