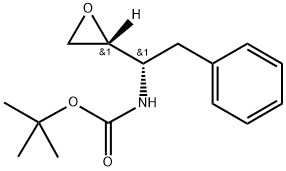
(2R,3S)-3-(tert-Butoxycarbonyl)amino-1,2-epoxy-4-phenylbutane synthesis
- Product Name:(2R,3S)-3-(tert-Butoxycarbonyl)amino-1,2-epoxy-4-phenylbutane
- CAS Number:98760-08-8
- Molecular formula:C15H21NO3
- Molecular Weight:263.34
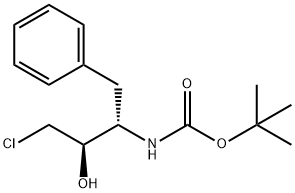
162536-40-5
72 suppliers
$6.00/250mg

98760-08-8
326 suppliers
$10.00/1g
Yield:98760-08-8 93%
Reaction Conditions:
with sodium hydroxide in water;isopropyl alcohol at 0; for 4 h;Product distribution / selectivity;
Steps:
4.4h
To (2R,3S)-3-tert-butoxycarbonylamino-1-chloro-2-hydroxy-4-phenylbutane (45.1 g) obtained in Example 3 were added isopropanol (120 ml) and water (45 ml), and the mixture was cooled to 0°C. 29%. Aqueous sodium hydroxide solution was added, and the mixture was stirred for 4 hours. Aqueous citric acid solution (a mixed solution of citric acid (6.73 g) and water (14 ml)) was added to the reaction mixture, and acetone (35 ml) and water (59.5 ml) were further added. A seed crystal of (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane was added, and this mixture was stirred for 1 hour. Water (200 ml) was added dropwise to the mixture over 1 hour, and the mixture was stirred overnight. The slurry solution was filtered, and the crystals were washed twice with aqueous acetone solution (a mixed solution of acetone (50 ml) and water (350 ml)). Wet crystals were dried under reduced pressure at room temperature to give (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane as white crystals (37.1 g, 100 wt%, yield 93%). As a result of HPLC analysis, it was found that the peak area ratio of the compound was 99.9%, and the diastereomer ((2S,3S)-form) was not detected.
References:
EP1777213,2007,A1 Location in patent:Page/Page column 21
![Carbamic acid, [(1S,2S)-3-(acetyloxy)-2-[(methylsulfonyl)oxy]-1-(phenylmethyl)propyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210305/GIF/259752-50-6.gif)
259752-50-6
0 suppliers
inquiry

98760-08-8
326 suppliers
$10.00/1g
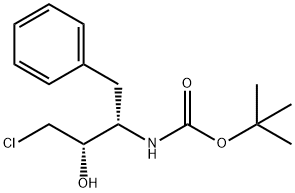
165727-45-7
136 suppliers
$20.00/250mg

98760-08-8
326 suppliers
$10.00/1g
![Carbamic acid, N-[(1S,2R)-2,3-dihydroxy-1-(phenylmethyl)propyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/99113-35-6.gif)
99113-35-6
4 suppliers
inquiry

98760-08-8
326 suppliers
$10.00/1g
![Carbamic acid, [(1S,2R)-2-(acetyloxy)-3-bromo-1-(phenylmethyl)propyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210305/GIF/200616-28-0.gif)
200616-28-0
0 suppliers
inquiry

98760-08-8
326 suppliers
$10.00/1g