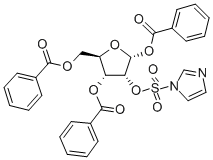
2-(1'-Imidazoylsulfonyl)-1,3,5-tri-O-benzoyl-alpha-D-ribofuranose synthesis
- Product Name:2-(1'-Imidazoylsulfonyl)-1,3,5-tri-O-benzoyl-alpha-D-ribofuranose
- CAS Number:97614-42-1
- Molecular formula:C29H24N2O10S
- Molecular Weight:592.57
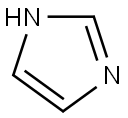
288-32-4
1017 suppliers
$5.00/25g
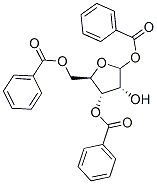
22224-41-5
288 suppliers
$6.00/250mg

97614-42-1
41 suppliers
$55.00/10g
Yield:97614-42-1 76%
Reaction Conditions:
Stage #1: 1,3,5-tri-O-benzoyl-α-D-ribofuranosidewith thionyl chloride;N,N-dimethyl-formamide in dichloromethane at -15 - 20;
Stage #2: 1H-imidazole in dichloromethane at 0 - 20;
Steps:
1
The synthesis of compound vii: The compound vi (2.81mmol) was dissolved in 13 ml dry dichloromethane and 3.5ml dry DMF, and SO2Cl2 (0.0079mmol) was slowly added while stirring the solution at -15°C. After it was stirred at -15°C for about 30 minutes, the solution was naturally warmed up to a room temperature, reacting for 3 hours. Then the solution was added with imidazole (0.0407mmol) for three times at 0°C. The mixed solution was stirred at a room temperature for 15 hours. The reaction solution was diluted with CH2Cl2 (26ml) added, and washed with ice water (35ml). The water layer was extracted with CH2Cl2. Then the organic layers were combined and then dried over anhydrous sodium sulfate for over 4 hours, and then drawn off under a reduced pressure to obtain light yellow syrup. The syrup was separated and purified by silica gel column (gradient elution, petroleum ether: acetone) to obtain the white solid compound vii (76.0%). 1H NMR(CDCl3) δppm: 7.00~8.10 (m, 15H, OBz), 6.71 (d, J=4.4Hz, 1H, H-1), 5.59 (dd, 1H, H-3), 5.25 (dd, 1H, H-2), 4.56~4.81 (m, 3H, H-4, H-5). M.p.128~129°C.
References:
EP2177527,2010,A1 Location in patent:Page/Page column 11