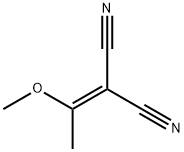
2-[3-(DIMETHYLAMINO)-1-METHOXY-2-PROPENYLIDENE]MALONONITRILE synthesis
- Product Name:2-[3-(DIMETHYLAMINO)-1-METHOXY-2-PROPENYLIDENE]MALONONITRILE
- CAS Number:95689-38-6
- Molecular formula:C9H11N3O
- Molecular Weight:177.2
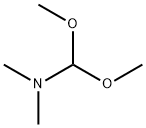
4637-24-5
607 suppliers
$5.00/25g
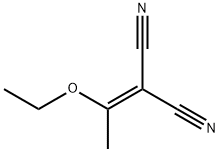
5417-82-3
118 suppliers
$9.00/1g
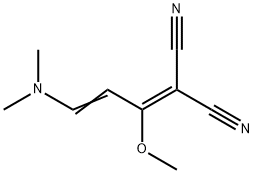
![2-[3-(DIMETHYLAMINO)-1-METHOXY-2-PROPENYLIDENE]MALONONITRILE](/CAS/GIF/95689-38-6.gif)
95689-38-6
71 suppliers
inquiry
![Propanedinitrile, 2-[3-(dimethylamino)-1-ethoxy-2-propen-1-ylidene]-](/CAS/20210305/GIF/98645-41-1.gif)
98645-41-1
1 suppliers
inquiry
Yield:-
Reaction Conditions:
at 100; for 1 h;
Steps:
41.41A
Example 41 A; 2-(3-dimethylamino-l-ethoxyallylidene)malononitrile:; 2-(l-ethoxyethylidene)malono-nitrile (110.0 g, 808 mmol) was dissolved in TV, /V- dimethylformamide dimethyl acetal (94%, 230 mL, 1.62 mol), and the reaction mixture was heated to reflux at 100 0C for 1 hr. Cooled down to room temperature. Solid was collected, and washed with cold methanol to give an orange solid product. The mother liquor was concentrated, and the solid was collected again, and washed with cold methanol. This procedure was repeated couple of times, and all the solid products were combined. The final residue was purified by a short column chromatography (SiO2, ethyl acetate). The combined yield of the reaction is 78% (120.0 g) as a mixture of 2-(3-dimethylamino-l-ethoxy- allylidene)malononitrile (73.5%) and 2-(3-dimethylamino-l-methoxyallylidene)malono- nitrile (26.5%). EPO
References:
ABBOTT LABORATORIES WO2006/81072, 2006, A1 Location in patent:Page/Page column 47-48

67-56-1
752 suppliers
$7.29/5ml-f

4637-24-5
607 suppliers
$5.00/25g

5417-82-3
118 suppliers
$9.00/1g
![2-[3-(DIMETHYLAMINO)-1-METHOXY-2-PROPENYLIDENE]MALONONITRILE](/CAS/GIF/95689-38-6.gif)
95689-38-6
71 suppliers
inquiry

67-56-1
752 suppliers
$7.29/5ml-f
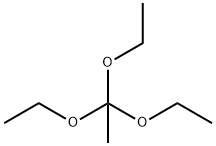
78-39-7
350 suppliers
$9.00/1g

109-77-3
433 suppliers
$10.00/5g
![2-[3-(DIMETHYLAMINO)-1-METHOXY-2-PROPENYLIDENE]MALONONITRILE](/CAS/GIF/95689-38-6.gif)
95689-38-6
71 suppliers
inquiry