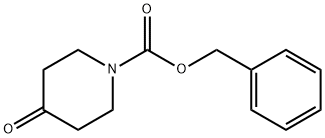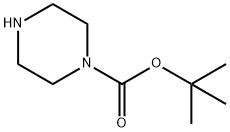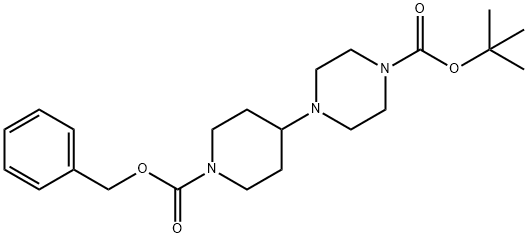
tert-butyl 4-(1-((benzyloxy)carbonyl)piperidin-4-yl)piperazine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(1-((benzyloxy)carbonyl)piperidin-4-yl)piperazine-1-carboxylate
- CAS Number:926904-28-1
- Molecular formula:C22H33N3O4
- Molecular Weight:403.52
Yield:926904-28-1 95%
Reaction Conditions:
with methanol;sodium tris(acetoxy)borohydride at 20; for 15 h;
Steps:
1.a
Example 1; Synthesis of 4-[1-(1 H-benzotriazole-5-carbonyl)-piperidin-4-yl]-piperazin-1- carbonic-acid-3,5-dichloro-benzylester 8a.; N-Boc-piperazin 1 (50.0 g, 268 mmol) and precursor 2 (70.0 g, 300 mmol) were given to MeOH (700 ml_), sodium-acetoxaboronhydride (70.0 g, 330 mmol) was added at RT. It was stirred for 15 h at RT. The main part of MeOH was removed in vacuo via a rotating evaporator. The residue was taken up in ethylacetate (400 mL) and washed with water (300 ml_). The aqueous phase was extracted with ethylacetate (200 mL), and the organic phases were pooled and dried with sodium sulphate. After filtration, the mixture was concentrated in vacuo until dryness and could be directly used without further processing (colourless oil 3, 101 g, 255 mmol, 95%).
References:
WO2010/115491,2010,A2 Location in patent:Page/Page column 84
205059-24-1
132 suppliers
$45.00/50mg
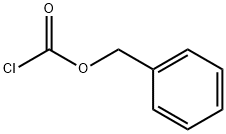
501-53-1
395 suppliers
$10.00/1g

926904-28-1
6 suppliers
inquiry