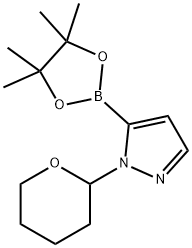
1-(Tetrahydropyran-2-yl)-1H-pyrazole-5-boronic acid pinacol ester synthesis
- Product Name:1-(Tetrahydropyran-2-yl)-1H-pyrazole-5-boronic acid pinacol ester
- CAS Number:903550-26-5
- Molecular formula:C14H23BN2O3
- Molecular Weight:278.15
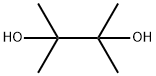
76-09-5
385 suppliers
$10.00/1g
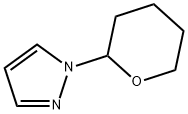
449758-17-2
176 suppliers
$5.00/1g

903550-26-5
224 suppliers
$6.00/1g
Yield:903550-26-5 78%
Reaction Conditions:
Stage #1: 2,3-dimethyl-2,3-butane diol;1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolewith n-butyllithium in tetrahydrofuran;hexanes at -78; for 0.0833333 h;
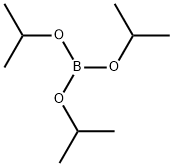
Stage #2: with Triisopropyl borate in tetrahydrofuran;hexanes at -78 - 20; for 17.5 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran;hexanes; pH=~ 7 - 8;
Steps:
7.14.1
l-(Tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazole (14.B.A). To a 500ml flask was added l-(tetrahydro-2H-pyran-2-yl)-lH- pyrazole 14.1.A and 100 ml of THF. The solution was cooled to -78 0C at which time butyllithium (52 ml, 83 mmole, 1.6M in hexanes) and pinacol (9.8 g, 83 mmole) were added. The reaction was stired for 5 minutes at -78 C and then triisopropyl borate (19 g, 103 mmole) was added. The reaction was slowly warmed to room temperature over 90 minutes and then stirred at room temperature for an additional 16 hours at which time the reaction was quenched with NH4Cl aq. (pH ~7-8), extracted with EtOAc, and purified by silica gel chromatography to give l-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)-lH-pyrazole 14.1.B (7.2 g, 38% yield).
References:
WO2010/93849,2010,A2 Location in patent:Page/Page column 63

76-09-5
385 suppliers
$10.00/1g
5419-55-6
380 suppliers
$12.00/5g

449758-17-2
176 suppliers
$5.00/1g

903550-26-5
224 suppliers
$6.00/1g
61676-62-8
321 suppliers
$11.19/5G

449758-17-2
176 suppliers
$5.00/1g

903550-26-5
224 suppliers
$6.00/1g