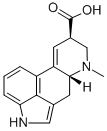
9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID synthesis
- Product Name:9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
- CAS Number:82-58-6
- Molecular formula:C16H16N2O2
- Molecular Weight:268.31
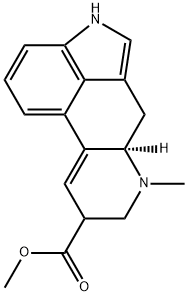
1159774-79-4
0 suppliers
inquiry

82-58-6
2 suppliers
inquiry
Yield:82-58-6 36%
Reaction Conditions:
with sodium hydroxide in ethanol;water at 35; for 2 h;
Steps:
(+)-Lysergic acid (1)
To a solution of a mixture of β,γ-unsaturated ester 25 (6.5 mg of the above material was used) in dichloromethane (0.15 ml) and dimethyl sulfide (0.15 ml) was added TFA (0.60 ml) at room temperature. After stirring for 1 h at 40 °C, the reaction mixture was diluted with toluene (1 ml). The solvents were removed under reduced pressure and the resultant residue was used in the next reaction without any purification. To a solution of the residue in acetonitrile (0.15 ml) was added pyridine (0.30 ml) at room temperature. After stirring at 50 °C for 20 min, the solvent was removed under reduced pressure and the resultant residue was purified with thin layer column chromatography on silica gel (7.5% methanol in chloroform) to give a diastereomeric mixture of methyl esters (4.3 mg, 42% in four steps, dr=1.8:1) as a brown oil. IR (film) 2950, 2850, 2799, 1732, 1558, 1540, 1507, 1455, 1435, 1068, 1057, 749 cm-1;HR-MS (ESI) 283.1460 (calcd for C17H19N2O2 283.1446). Major isomer: 1HNMR (400 MHz, CDCl3) δ 7.92 (br s, 1H), 7.24-7.16 (m, 3H), 6.92 (s, 1H),6.60 (s, 1H), 3.78 (s, 3H), 3.72 (m, 1H), 3.53 (dd, J=15.1, 5.5 Hz, 1H), 3.31(m, 1H), 3.20 (m, 1H), 2.78-2.69 (m, 2H), 2.62 (s, 3H). Minor isomer: 1HNMR (400 MHz, CDCl3) δ 7.92 (br s, 1H), 7.24-7.16 (m, 3H), 6.90 (s, 1H),6.56 (d, J=4.1 Hz, 1H), 3.72 (s, 3H), 3.43 (dd, J=14.8, 5.2 Hz, 1H), 3.36 (dd,J=11.4, 3.7 Hz, 1H), 3.31 (m, 1H), 3.20 (m, 1H), 2.78-2.69 (m, 2H), 2.57(s, 3H). The 1H NMR spectrum was in good accordance with those reported by Ohno and co-workers. 20 To solution of the mixture of methyl esters (8.3 mg,0.0293 mmol) in ethanol (0.30 ml) was added 1 N aqueous sodium hydroxide (0.30 ml). The reaction mixture was stirred at 35 °C for 2 h. One normal of hydrochloric acid solution was used to carefully adjust the pH to 6 and stirredat 0 °C for 2 h while a solid material was formed. The precipitate was filtered offand washed with cold water and acetone to give lysergic acid (1, 2.8mg, 36%) as a pale red-brown solid. [α]D20.9 +46.1 (c 0.14, pyridine); lit.16 [α]D20 +40(c 0.50, pyridine); IR (film) 3243, 3207, 2921, 2850, 1597, 1455, 1375 cm- 1; 1HNMR (400 MHz, C5D5N) δ 11.71 (br s, 1H), 7.45 (d, J=7.8 Hz, 1H), 7.43 (d, J=7.8 Hz, 1H), 7.30 (dd, J=7.8, 7.8 Hz, 1H), 7.21-7.19 (m, 2H), 4.06(m, 1H), 3.64 (dd, J=14.6, 5.5 Hz, 1H), 3.54 (dd, J=14.6, 5.0 Hz, 1H), 3.29(m, 1H), 2.96-2.88 (m, 2H), 2.52 (s, 3H); 13C NMR (100 MHz, C5D5N) δ175.1 (C), 136.7 (C), 135.9 (C), 128.9 (C), 127.3 (C), 123.3 (CH), 120.1 (CH),119.8 (CH), 112.2 (CH), 110.5 (C), 110.5 (CH), 63.8 (CH), 56.0 (CH2), 43.9(CH3), 43.3 (CH), 27.9 (CH2), HR-MS (ESI) 291.1111 (calcd forC16H16N2NaO2 291.1109).
References:
Kanno, Rentaro;Yokoshima, Satoshi;Kanai, Motomu;Fukuyama, Tohru [Journal of Antibiotics,2018,vol. 71,# 2,p. 240 - 247]
![Formamide, N-[(9,10-didehydro-6-methylergolin-8-ylidene)[(4-methylphenyl)sulfonyl]methyl]-](/CAS/20210305/GIF/764710-82-9.gif)
764710-82-9
0 suppliers
inquiry

82-58-6
2 suppliers
inquiry
![Benz[cd]indol-5(1H)-one, 4-bromo-3,4-dihydro-](/CAS/20210111/GIF/764710-89-6.gif)
764710-89-6
0 suppliers
inquiry

82-58-6
2 suppliers
inquiry
![Benz[cd]indol-5(1H)-one, 3,4-dihydro-4-[methyl(2-oxopropyl)amino]-](/CAS/20210305/GIF/764710-84-1.gif)
764710-84-1
0 suppliers
inquiry

82-58-6
2 suppliers
inquiry
![Benz[cd]indol-5(1H)-one, 3,4-dihydro-4-[methyl[(2-methyl-1,3-dioxolan-2-yl)methyl]amino]-](/CAS/20210305/GIF/764710-85-2.gif)
764710-85-2
0 suppliers
inquiry

82-58-6
2 suppliers
inquiry