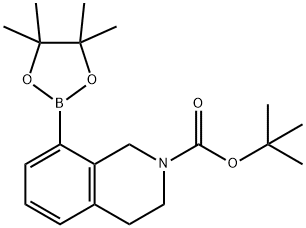
2(1H)-ISOQUINOLINECARBOXYLIC ACID, 3,4-DIHYDRO-8-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-, 1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:2(1H)-ISOQUINOLINECARBOXYLIC ACID, 3,4-DIHYDRO-8-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-, 1,1-DIMETHYLETHYL ESTER
- CAS Number:893566-73-9
- Molecular formula:C20H30BNO4
- Molecular Weight:359.27
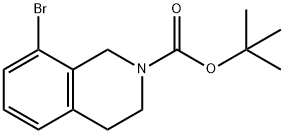
893566-75-1
70 suppliers
$11.00/100mg
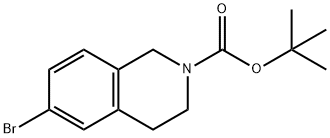
893566-74-0
109 suppliers
$28.00/250mg
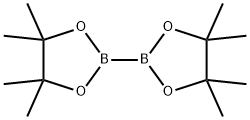
73183-34-3
581 suppliers
$6.00/5g
893566-72-8
82 suppliers
$59.00/2mg

893566-73-9
18 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in N,N-dimethyl-formamide at 85; for 4 h;
Steps:
1.6
A solution of tert-butyl 6-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate and tert-butyl 8-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate (19.5 mmol), bis(pinacolato)diboron (21.4 mmol) and potassium acetate (61 mmol) in DMF (100 mL) was degassed. To this solution was added PdCl2dppf (1:1 complex with DCM, 0.8 mmol). The reaction mixture was heated at 85° C. for 4 hr and then allowed to cool to room temperature and diluted with EtOAc. The solution was washed with water and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography to give tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate and tert-butyl 8-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate. LCMS: (FA) ES+360 (for each).
References:
US2008/171754,2008,A1 Location in patent:Page/Page column 102