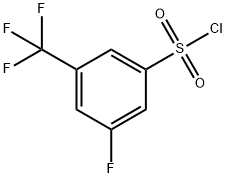
3-FLUORO-5-(TRIFLUOROMETHYL)BENZENESULPHONYL CHLORIDE synthesis
- Product Name:3-FLUORO-5-(TRIFLUOROMETHYL)BENZENESULPHONYL CHLORIDE
- CAS Number:886499-99-6
- Molecular formula:C7H3ClF4O2S
- Molecular Weight:262.61
454-67-1
149 suppliers
$15.00/1g

886499-99-6
33 suppliers
$89.70/250mg
Yield:886499-99-6 26%
Reaction Conditions:
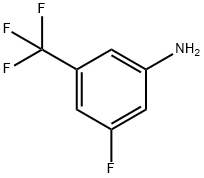
Stage #1:3-fluoro-5-trifluoromethylaniline with hydrogenchloride;trifluoroacetic acid;sodium nitrite in water at 0;
Stage #2: with sulfur dioxide;sulphurous acid;acetic acid;copper(l) chloride;copper dichloride in water at 0 - 20;
Steps:
Preparation of 3-fluoro-5-trifluoromethyl-benzenesulfonyl chloride; A mixture of 3-fluoro-5-trifluoromethyl-phenylamine (9.7 g, 54 mmol) in trifluoroacetic acid (100 mL) was cooled at 0° C. To the mixture was slowly added concentrated hydrochloric acid (10 mL), followed by a solution of sodium nitrite (4.7 g, 68 mmol) in water (5 mL) dropwise over 20 minutes. The mixture was stirred for another 10 minutes at 0° C., and then poured into a stirred mixture of acetic acid (120 mL), sulfurous acid (0.94 N aqueous sulfur dioxide solution, 120 mL), copper(II) chloride (9.2 g, 93 mmol) and copper(I) chloride (100 mg, 0.74 mmol) at 0° C. The resulting reaction mixture was allowed to warm to room temperature and stirred for 15 hours. Water (200 mL) was added, and the resulting mixture was extracted with ethyl acetate (100 mL×3). The combined organic layers were dried over sodium sulfate, filtered through a glass funnel and concentrated in vacuo. The residue was purified by column chromatography (20% ethyl acetate in petroleum ether) to afford 3-fluoro-5-trifluoromethyl-benzenesulfonyl chloride (3.7 g, 26%) as a white solid (reference: Cherney, R. J. et al., J. Med. Chem. 46 (2003) 1811). 1H NMR (400 MHz, CDCl3) δ ppm 8.15 (s, 1H); 7.97-7.99 (d, J=4.0 Hz, 1H); 7.74-7.76 (d, J=4.0 Hz, 1H).
References:
Firooznia, Fariborz;Gillespie, Paul;Goodnow, JR., Robert Alan;Lin, Tai-An;Sidduri, Achyutharao;So, Sung-Sau;Tan, Jenny US2010/41713, 2010, A1 Location in patent:Page/Page column 21