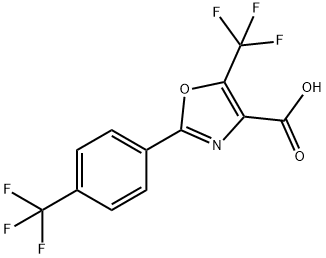
5-(TRIFLUOROMETHYL)-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-OXAZOLE-4-CARBOXYLICACID synthesis
- Product Name:5-(TRIFLUOROMETHYL)-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-OXAZOLE-4-CARBOXYLICACID
- CAS Number:886497-47-8
- Molecular formula:C12H5F6NO3
- Molecular Weight:325.16
![5-(TRIFLUOROMETHYL)-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-OXAZOLE-4-CARBOXYLICACID](/CAS/GIF/886497-47-8.gif)
886497-47-8
20 suppliers
$60.00/250mg
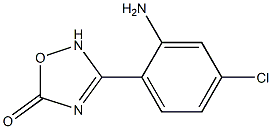
697278-41-4
0 suppliers
inquiry
![4-Oxazolecarboxamide, N-[5-chloro-2-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)phenyl]-5-(trifluoromethyl)-2-[4-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/1083418-59-0.gif)
1083418-59-0
0 suppliers
inquiry
Yield:1083418-59-0 45%
Reaction Conditions:
Stage #1: 5-(trifluoromethyl)-2-[4-(trifluoromethyl)phenyl]-1,3-oxazole-4-carboxylic acidwith dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane; for 0.166667 h;
Stage #2: 3-(2-amino-4-chlorophenyl)-4H-[1,2,4]oxadiazol-5-one in dichloromethane at 20;
Steps:
δ-Trifluoromethyl^-^-trifluoromethyl-phenylj-oxazole^-carboxylic acid [5-chloro-2-(5- oxo-4,5-dihydro-[1,2,4]oxadiazol-3-yl)-phenyl]-amide (16)To a suspension of 5-(trifluoromethyl)-2-[4-(trifluoromethyl)phenyl]-1 ,3- oxazole-4-carboxylic acid (0.25 g, 1 eq) in DCM (15 ml), EDC HCI (0.295 g, 2 eq) and DMAP (0.282 g, 3 eq) are added. The resulting solution is stirred for 10 min and 3-(2- amino-4-chloro-phenyl)-4H-[1 ,2,4]oxadiazol-5-one (0.166 g, 1 eq) (prepared as described by Valgeirsson et al. in Journal of Medicinal Chemistry 2004 47 (27) 6948- 6957) is added. The solution is stirred at room temperature overnight, diluted with DCM (20 ml), washed with 1.5 N HCI (30 ml) and water (30 ml), dried and evaporated to dryness, to give a pure white solid (0.176 g, 45% yield). LC-ESI-HRMS of [M-H]- shows 517,0135 Da. CaIc. 517,013827 Da, dev. -0,6 ppm.
References:
WO2008/138917,2008,A1 Location in patent:Page/Page column 30
![5-(TRIFLUOROMETHYL)-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-OXAZOLE-4-CARBOXYLICACID](/CAS/GIF/886497-47-8.gif)
886497-47-8
20 suppliers
$60.00/250mg
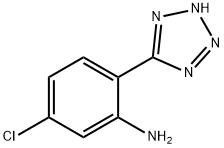
54013-18-2
29 suppliers
$27.00/1g
![4-Oxazolecarboxamide, N-[5-chloro-2-(2H-tetrazol-5-yl)phenyl]-5-(trifluoromethyl)-2-[4-(trifluoromethyl)phenyl]-](/CAS/20200611/GIF/1083418-61-4.gif)
1083418-61-4
0 suppliers
inquiry